Calculate the Molar Mass Magnesium chloride Ammonium sulfate









- Slides: 15

Calculate the Molar Mass Magnesium chloride Ammonium sulfate Answers: 95. 21 g/mol; 132. 17 g/mol

Convert to Moles 120. 4 grams of He 30. 1 grams of Cu

Convert to Moles 3. 61 grams of Fe 100 grams of Ar

Convert to Moles 1 gram of S 4. 5 grams of Zn

Convert to Moles 24 grams of C 59. 3 grams of Sn

Convert to Moles 98. 9 grams Na 5000 grams of K

Convert to Mass 10. 0 moles Na 2. 20 moles Sn

Convert to Mass 5. 00 moles Ag 3. 0 x 10 -4 moles Au

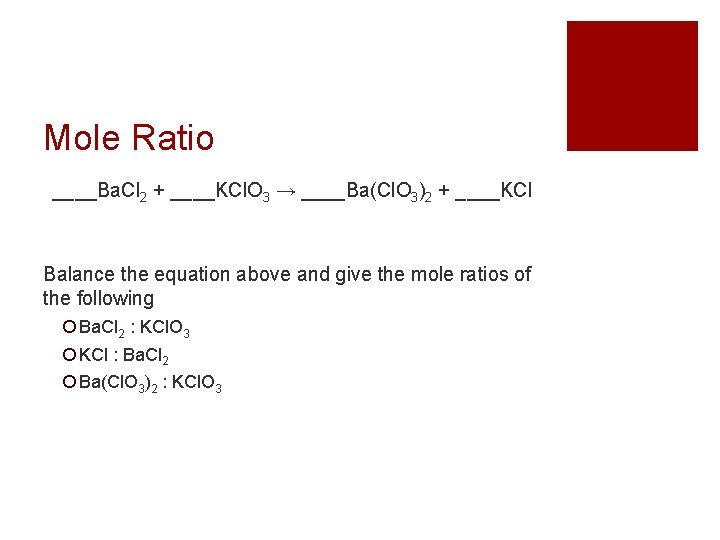
Mole Ratio ____Ba. Cl 2 + ____KCl. O 3 → ____Ba(Cl. O 3)2 + ____KCl Balance the equation above and give the mole ratios of the following Ba. Cl 2 : KCl. O 3 KCl : Ba. Cl 2 Ba(Cl. O 3)2 : KCl. O 3

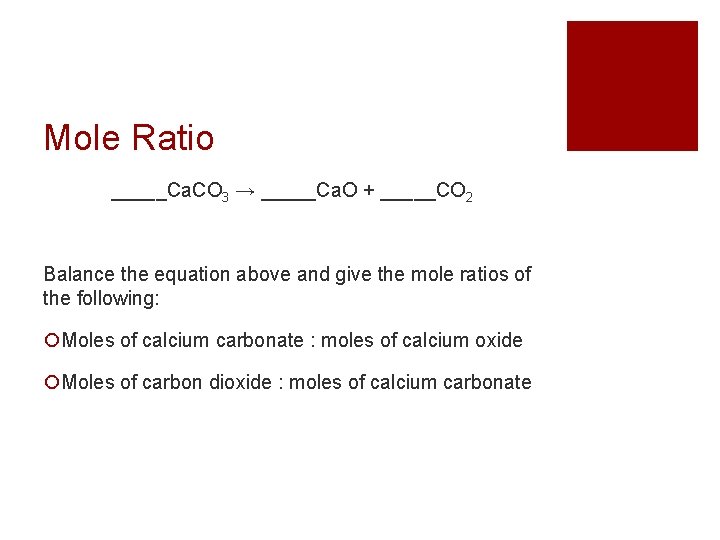
Mole Ratio _____Ca. CO 3 → _____Ca. O + _____CO 2 Balance the equation above and give the mole ratios of the following: Moles of calcium carbonate : moles of calcium oxide Moles of carbon dioxide : moles of calcium carbonate

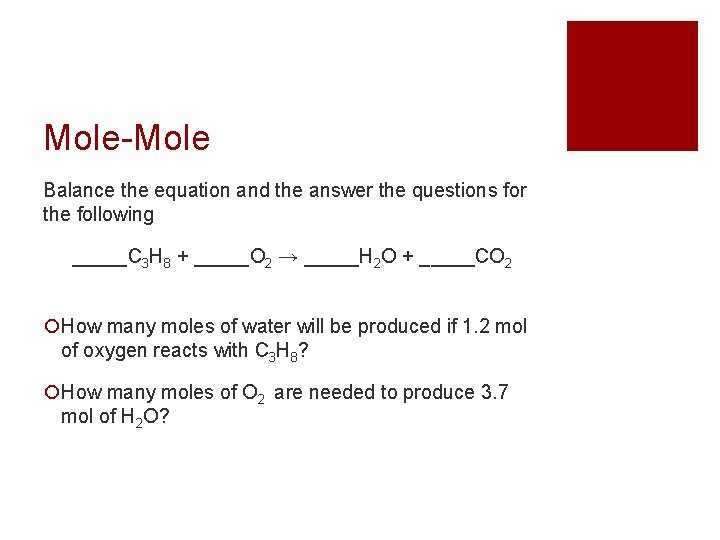
Mole-Mole Balance the equation and the answer the questions for the following _____C 3 H 8 + _____O 2 → _____H 2 O + _____CO 2 How many moles of water will be produced if 1. 2 mol of oxygen reacts with C 3 H 8? How many moles of O 2 are needed to produce 3. 7 mol of H 2 O?

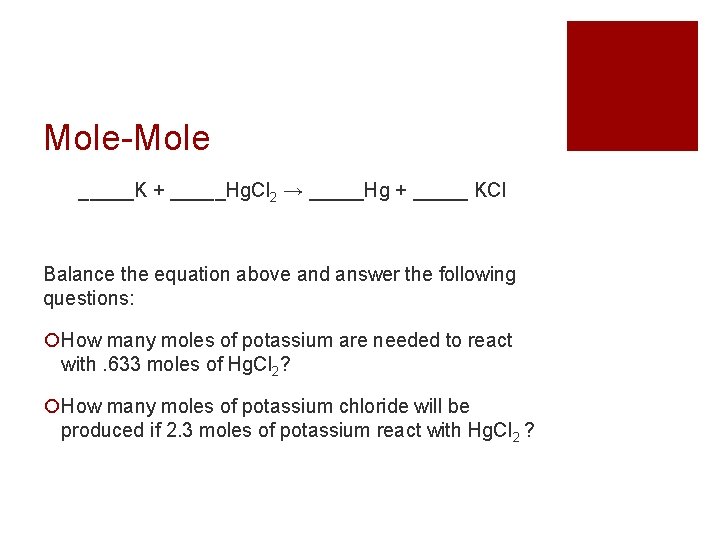
Mole-Mole _____K + _____Hg. Cl 2 → _____Hg + _____ KCl Balance the equation above and answer the following questions: How many moles of potassium are needed to react with. 633 moles of Hg. Cl 2? How many moles of potassium chloride will be produced if 2. 3 moles of potassium react with Hg. Cl 2 ?

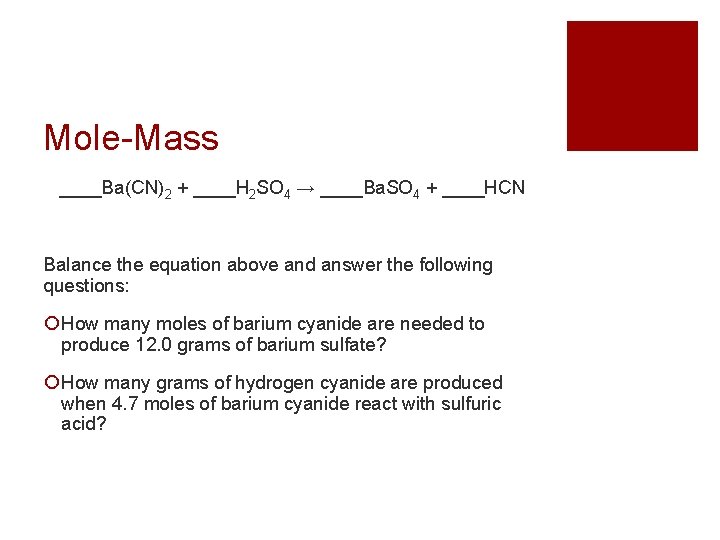
Mole-Mass ____Ba(CN)2 + ____H 2 SO 4 → ____Ba. SO 4 + ____HCN Balance the equation above and answer the following questions: How many moles of barium cyanide are needed to produce 12. 0 grams of barium sulfate? How many grams of hydrogen cyanide are produced when 4. 7 moles of barium cyanide react with sulfuric acid?

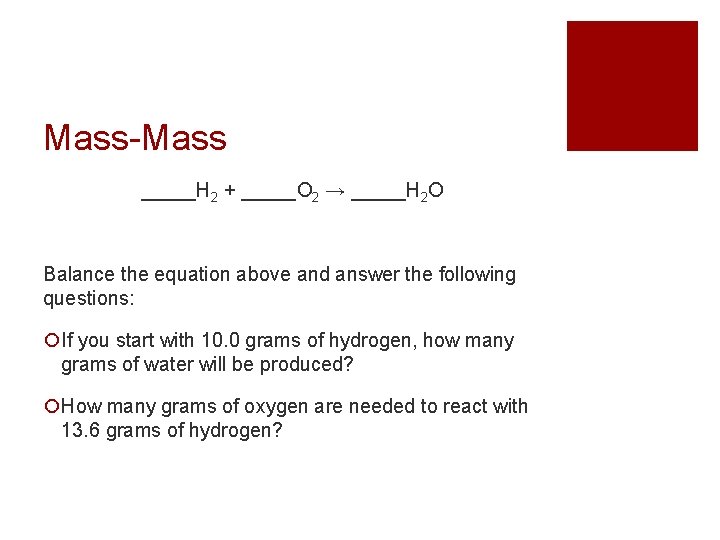
Mass-Mass _____H 2 + _____O 2 → _____H 2 O Balance the equation above and answer the following questions: If you start with 10. 0 grams of hydrogen, how many grams of water will be produced? How many grams of oxygen are needed to react with 13. 6 grams of hydrogen?

Mass-Mass ___ HCl + ___ Na 2 SO 4 ___ Na. Cl + ___ H 2 SO 4 Balance the equation above and answer the following questions: How many grams of hydrochloric acid are needed to produce 20 grams of sulfuric acid? How many grams of sodium chloride will be produced if 5. 3 grams of sodium sulfate reacts with hydrochloric acid?
 Ammonium sulfate precipitation
Ammonium sulfate precipitation Magnesium carbonate cation and anion
Magnesium carbonate cation and anion Nitric acid and calcium carbonate
Nitric acid and calcium carbonate Complications of asthma
Complications of asthma Tocolytic
Tocolytic Acute fulminating preeclampsia
Acute fulminating preeclampsia Magnesium sulfate toxicity level
Magnesium sulfate toxicity level Side effects of magnesium sulfate in pregnancy
Side effects of magnesium sulfate in pregnancy Summary of buffer solution
Summary of buffer solution Xxxh3
Xxxh3 Definition of buffering capacity
Definition of buffering capacity Ammonium chloride uses
Ammonium chloride uses Ammonium chloride reversible reaction
Ammonium chloride reversible reaction Gerald da roza
Gerald da roza Calcium phosphate calculi
Calcium phosphate calculi Magnesium ammonium phosphate stones
Magnesium ammonium phosphate stones