Practical organic chemistry Ammonium salts and amides Ammonium
- Slides: 12

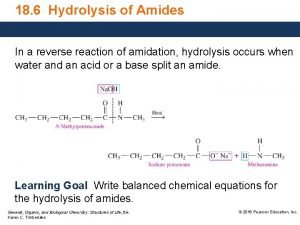
Practical organic chemistry Ammonium salts and amides

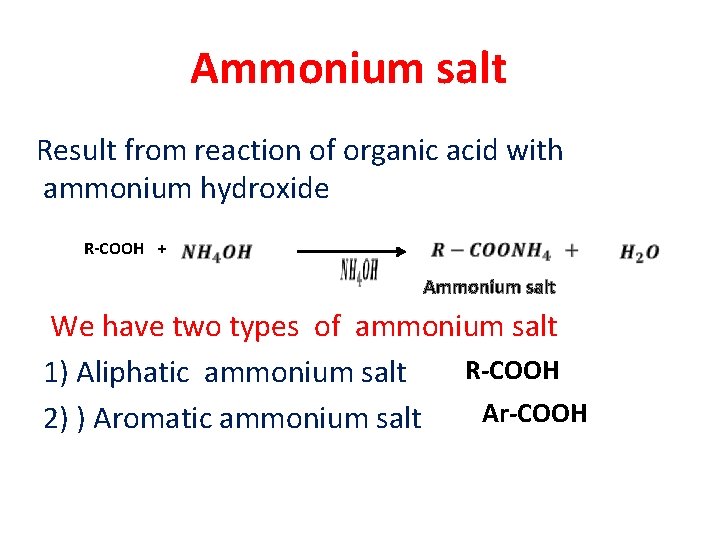
Ammonium salt Result from reaction of organic acid with ammonium hydroxide R-COOH + Ammonium salt We have two types of ammonium salt R-COOH 1) Aliphatic ammonium salt Ar-COOH 2) ) Aromatic ammonium salt

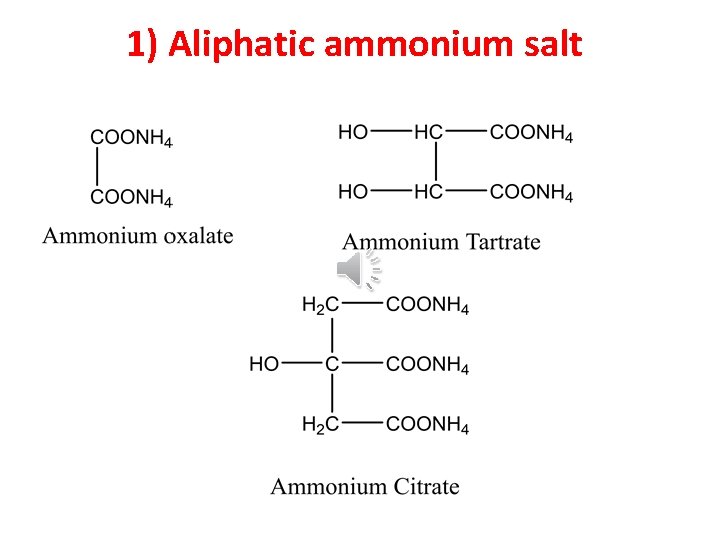
1) Aliphatic ammonium salt

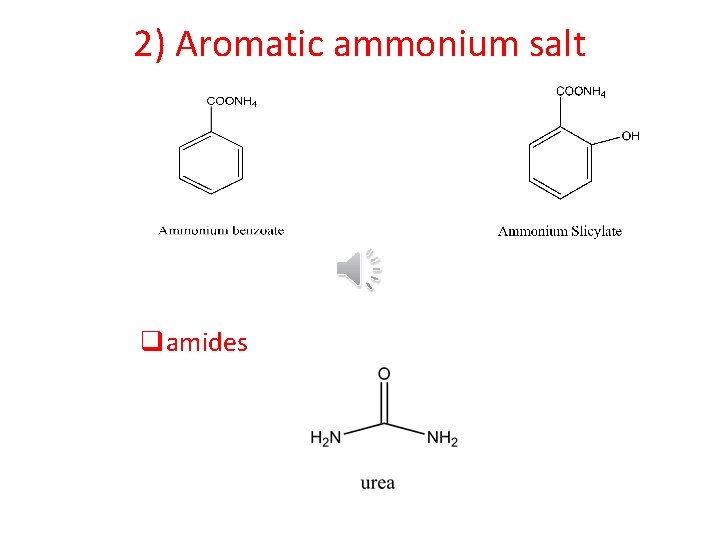
2) Aromatic ammonium salt qamides

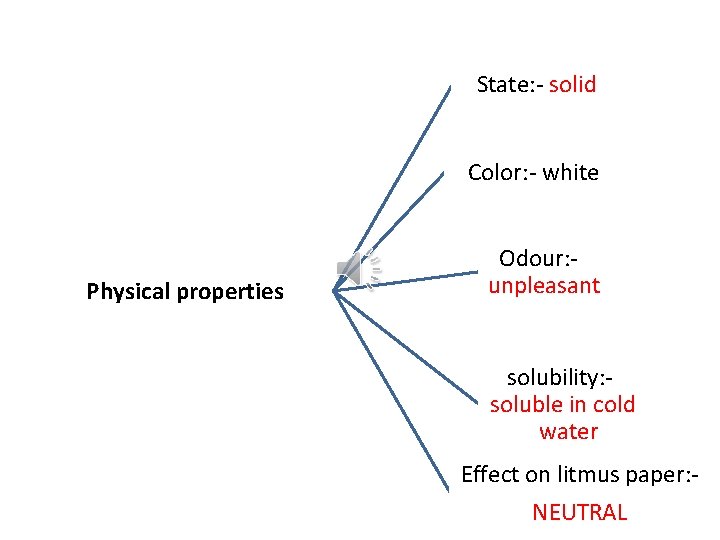
State: - solid Color: - white Physical properties Odour: unpleasant solubility: soluble in cold water Effect on litmus paper: NEUTRAL

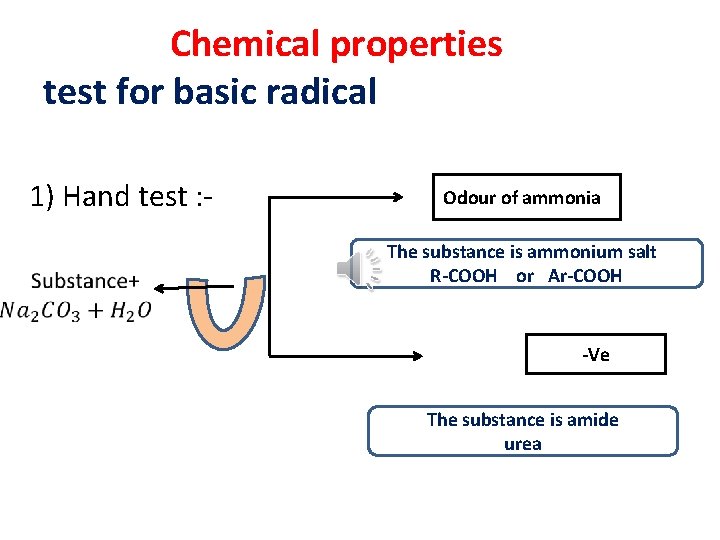
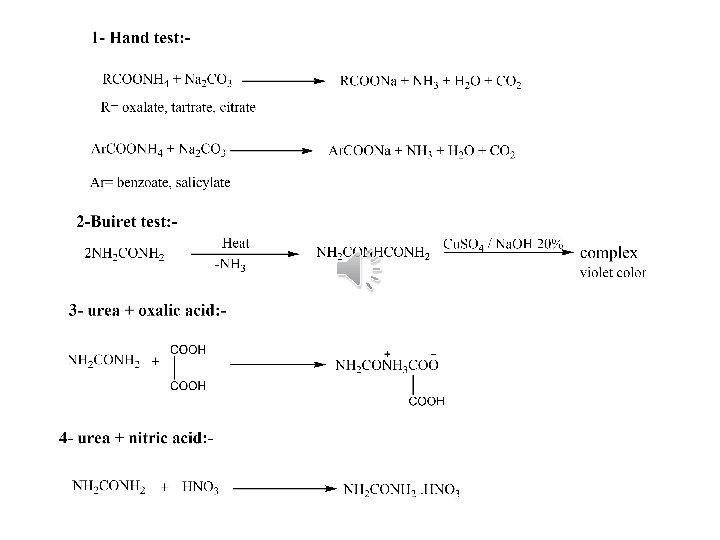
Chemical properties test for basic radical 1) Hand test : - Odour of ammonia The substance is ammonium salt R-COOH or Ar-COOH -Ve The substance is amide urea

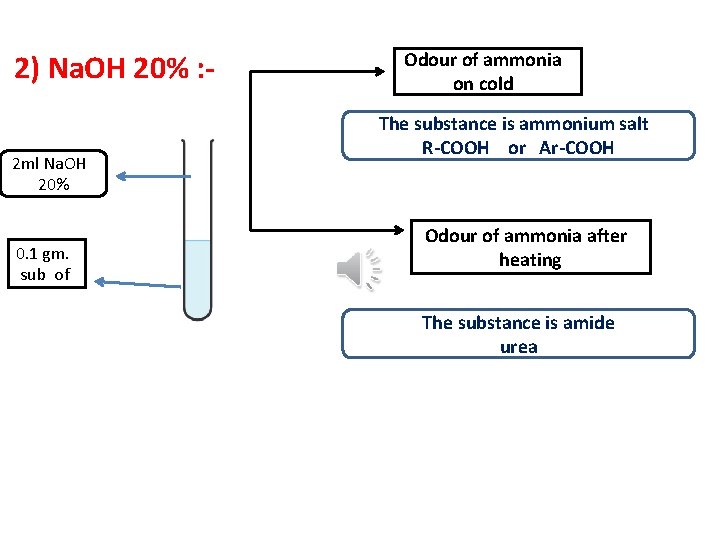
2) Na. OH 20% : 2 ml Na. OH 20% 0. 1 gm. sub of Odour of ammonia on cold The substance is ammonium salt R-COOH or Ar-COOH Odour of ammonia after heating The substance is amide urea

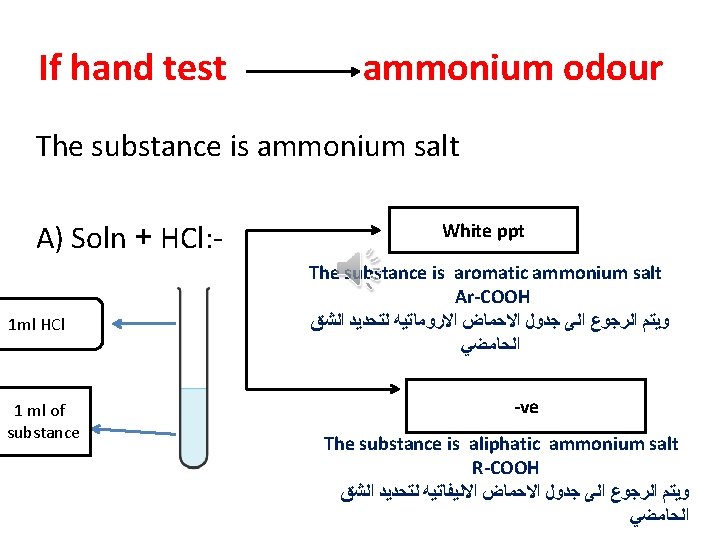
If hand test ammonium odour The substance is ammonium salt A) Soln + HCl: 1 ml HCl 1 ml of substance White ppt The substance is aromatic ammonium salt Ar-COOH ﻭﻳﺘﻢ ﺍﻟﺮﺟﻮﻉ ﺍﻟﻰ ﺟﺪﻭﻝ ﺍﻻﺣﻤﺎﺽ ﺍﻻﺭﻭﻣﺎﺗﻴﻪ ﻟﺘﺤﺪﻳﺪ ﺍﻟﺸﻖ ﺍﻟﺤﺎﻣﻀﻲ -ve The substance is aliphatic ammonium salt R-COOH ﻭﻳﺘﻢ ﺍﻟﺮﺟﻮﻉ ﺍﻟﻰ ﺟﺪﻭﻝ ﺍﻻﺣﻤﺎﺽ ﺍﻻﻟﻴﻔﺎﺗﻴﻪ ﻟﺘﺤﺪﻳﺪ ﺍﻟﺸﻖ ﺍﻟﺤﺎﻣﻀﻲ

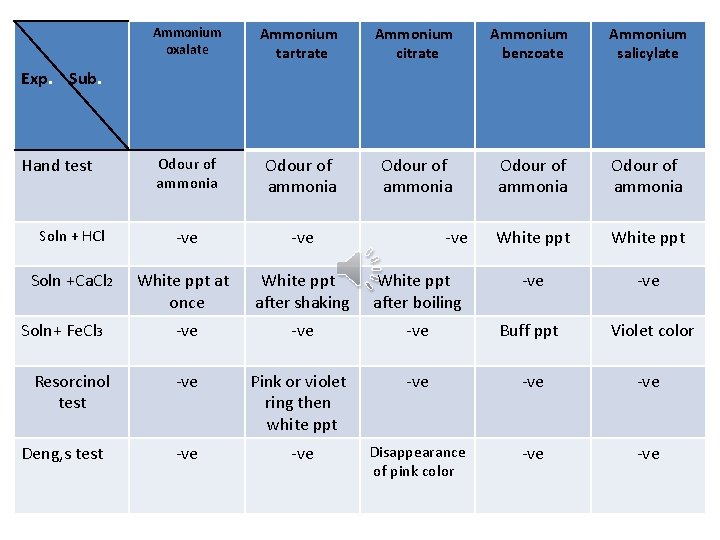
Ammonium oxalate Ammonium tartrate Ammonium citrate Ammonium benzoate Ammonium salicylate Odour of ammonia Odour of ammonia Soln + HCl -ve White ppt Soln +Ca. Cl 2 White ppt at once White ppt after shaking White ppt after boiling -ve -ve -ve Buff ppt Violet color -ve Pink or violet ring then white ppt -ve -ve -ve Disappearance of pink color -ve Exp. Sub. Hand test Soln+ Fe. Cl 3 Resorcinol test Deng, s test -ve

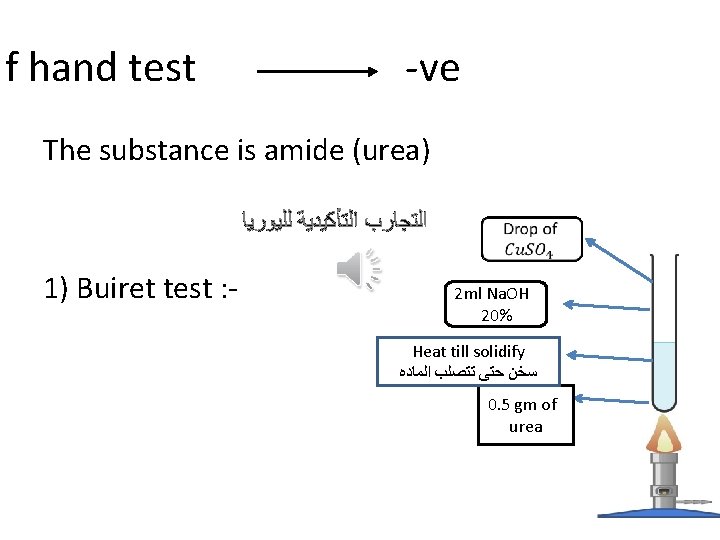
If hand test -ve The substance is amide (urea) ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﺘﺄﻜﻴﺪﻳﺔ ﻟﻠﻴﻮﺭﻳﺎ 1) Buiret test : - 2 ml Na. OH 20% Heat till solidify ﺳﺨﻦ ﺣﺘﻰ ﺗﺘﺼﻠﺐ ﺍﻟﻤﺎﺩﻩ 0. 5 gm of urea

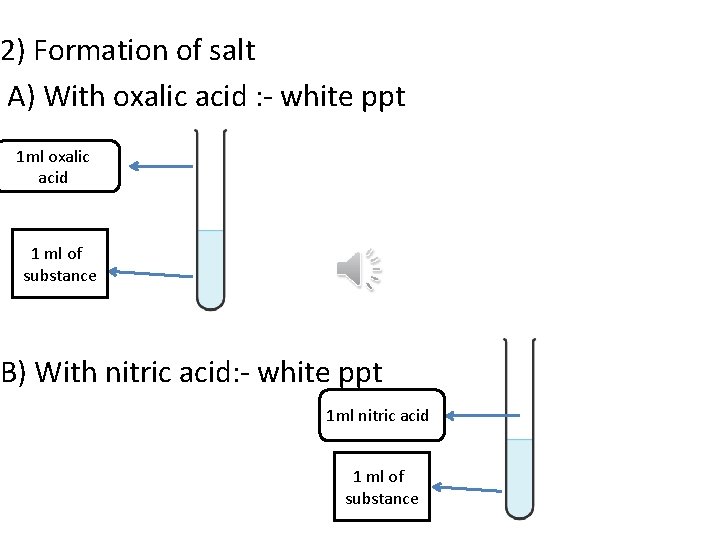
2) Formation of salt A) With oxalic acid : - white ppt 1 ml oxalic acid 1 ml of substance B) With nitric acid: - white ppt 1 ml nitric acid 1 ml of substance