Atoms Elements and Compounds Chapter 7 Elements and








- Slides: 21


Atoms, Elements, and Compounds

Chapter 7: Elements and the Periodic Table • 7. 1 The Periodic Table • 7. 2 Properties of the Elements

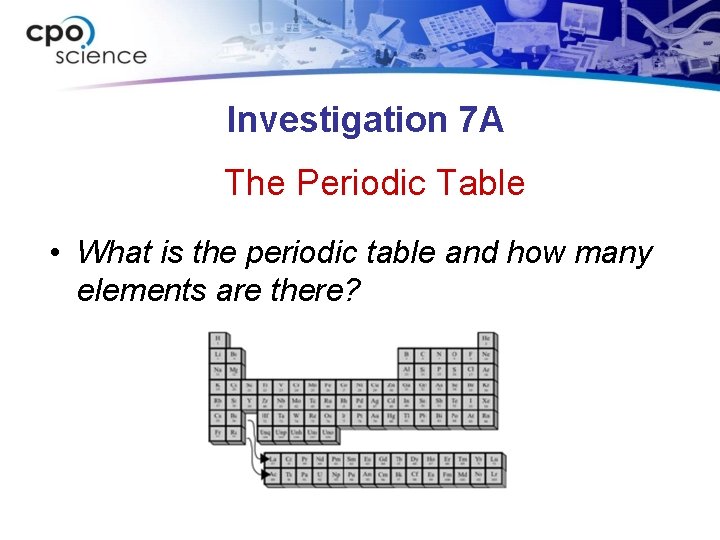
Investigation 7 A The Periodic Table • What is the periodic table and how many elements are there?

7. 1 Physical properties • Characteristics that you can see through direct observation are called physical properties. • Physical properties include color, texture, density, brittleness, and state (solid, liquid, or gas). • Melting point, boiling point, and specific heat are also physical properties.

7. 1 Chemical properties • Properties that can only be observed when one substance changes into a different substance are called chemical properties. • Any change that transforms one substance into a different substance is called a chemical change.

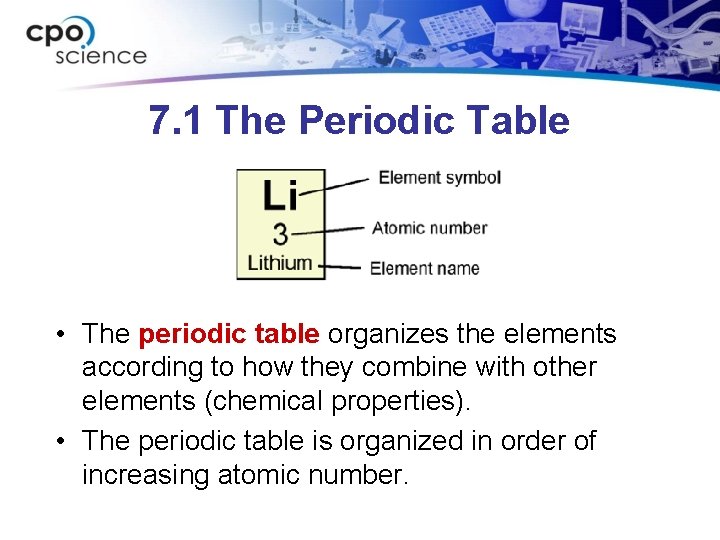
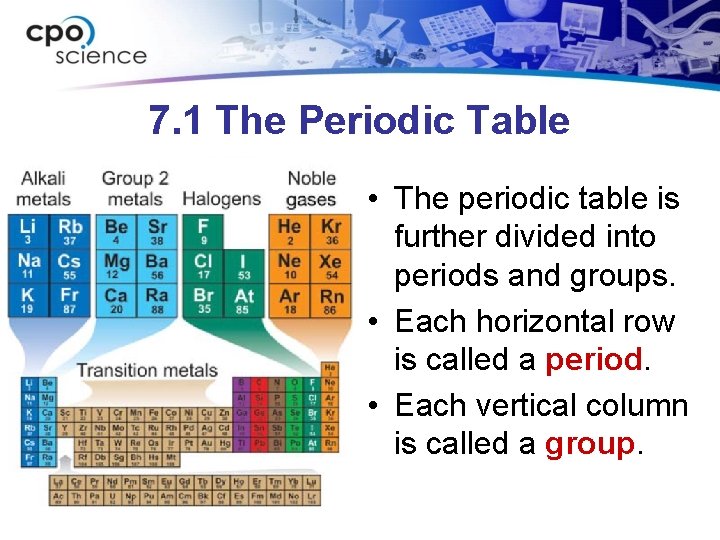
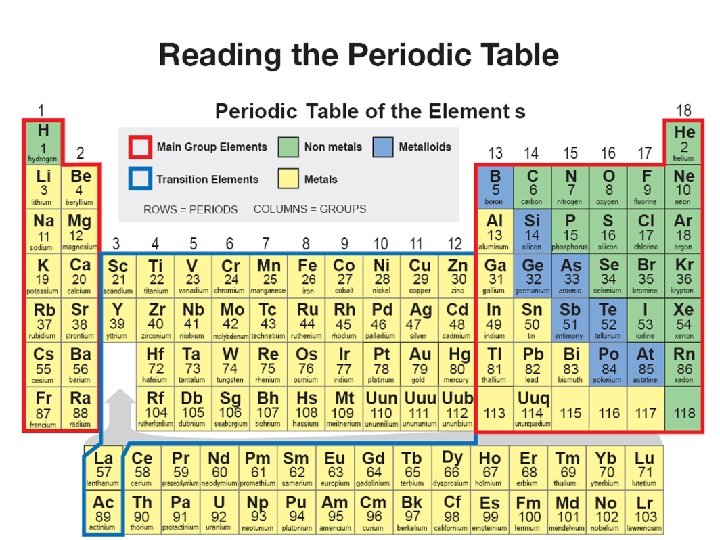
7. 1 The Periodic Table • The periodic table organizes the elements according to how they combine with other elements (chemical properties). • The periodic table is organized in order of increasing atomic number.

7. 1 The Periodic Table • The periodic table is further divided into periods and groups. • Each horizontal row is called a period. • Each vertical column is called a group.

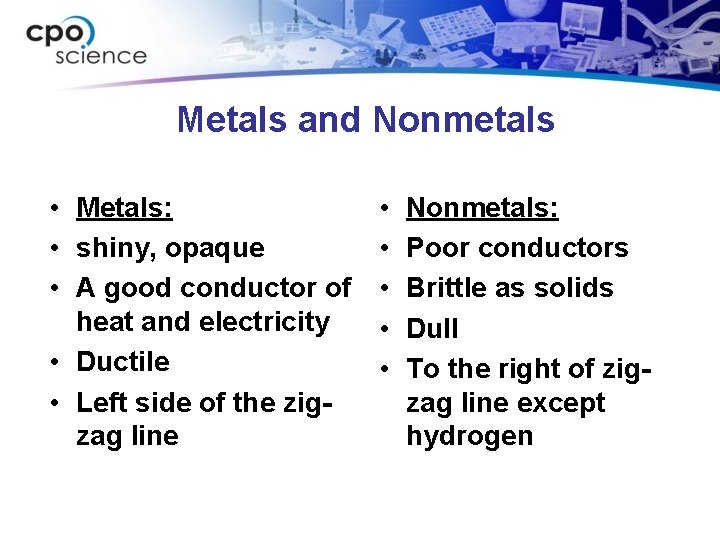
Metals and Nonmetals • Metals: • shiny, opaque • A good conductor of heat and electricity • Ductile • Left side of the zigzag line • • • Nonmetals: Poor conductors Brittle as solids Dull To the right of zigzag line except hydrogen

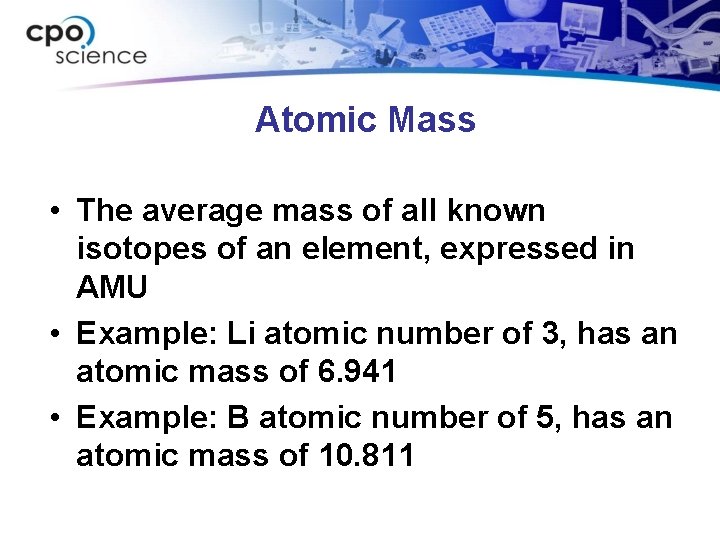
Atomic Mass • The average mass of all known isotopes of an element, expressed in AMU • Example: Li atomic number of 3, has an atomic mass of 6. 941 • Example: B atomic number of 5, has an atomic mass of 10. 811


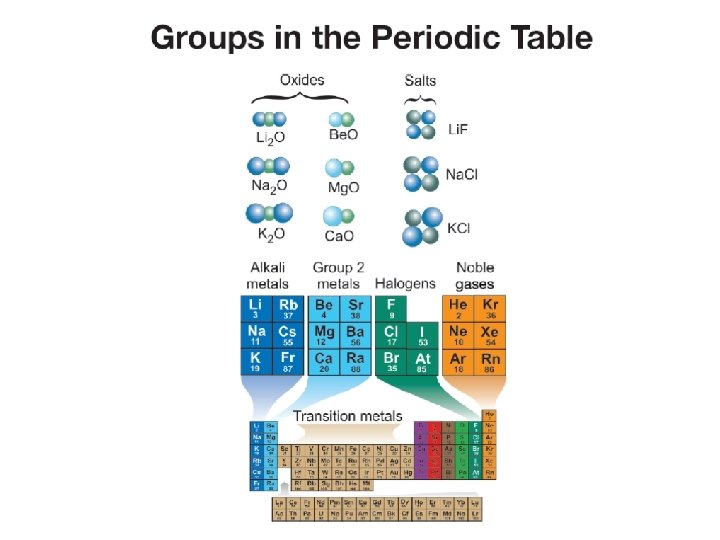
Groups of the Periodic Table Alkali Metals: • (group #1) Includes elements Li, Na, K, Rb, Cs • Soft, silvery metals that are highly reactive. • Each combine in a two to one ratio with oxygen • Have one Valence electron

Alkaline Earth Metals • (group #2) metals: • Include elements Be, Mg, Ca, Sr, Ba • These are less reactive than the group 1 elements • Form oxides with oxygen in a ratio of 1 to 1. • Have two valence electrons

Halogens • • • Group 17 elements Nonmetals Toxic gases or liquids Include elements F, Cl, Br, I Form salts with alkali metals 7 valence electrons

Noble Gases • Group 18 • Include elements He, ne, Ar Kr, Xe • Are completely inert, un-reactive in nature • All gases • Full shell of 8 electrons

Transition Metals • • • Groups 3 -12 Middle of the table Include Ti, Fe, Cu Good conductors Have either 1 or 2 valence electrons Considered reactive, but less than groups 1 and 2


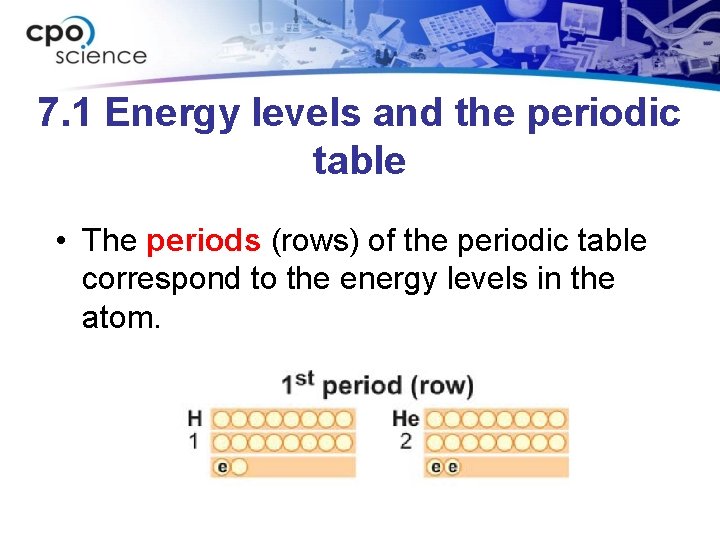
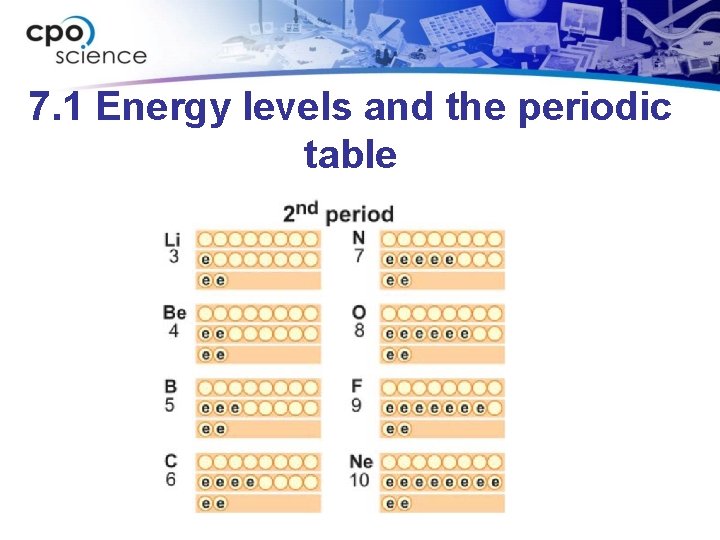
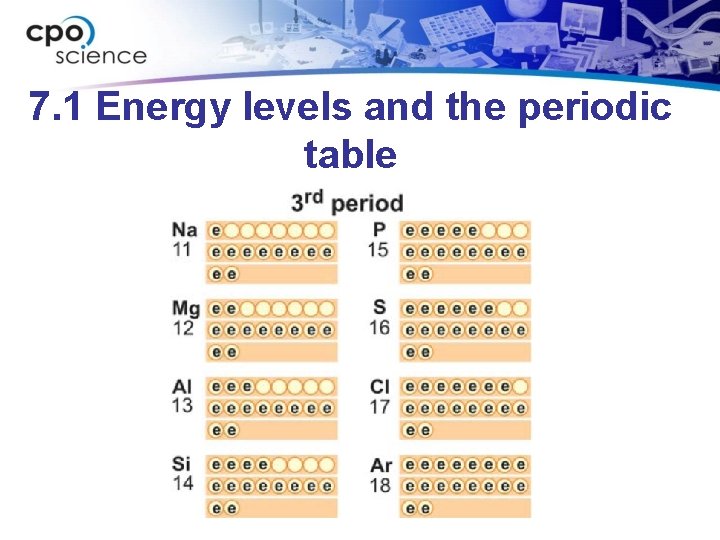
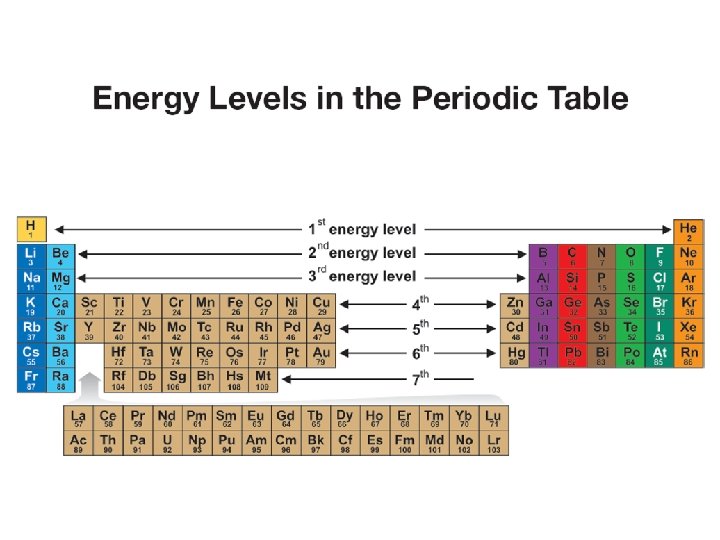
7. 1 Energy levels and the periodic table • The periods (rows) of the periodic table correspond to the energy levels in the atom.

7. 1 Energy levels and the periodic table

7. 1 Energy levels and the periodic table
