Atomic Structure What is an atom Atom the



- Slides: 16

Atomic Structure

What is an atom? • Atom: the smallest unit of matter that retains the identity of the substance

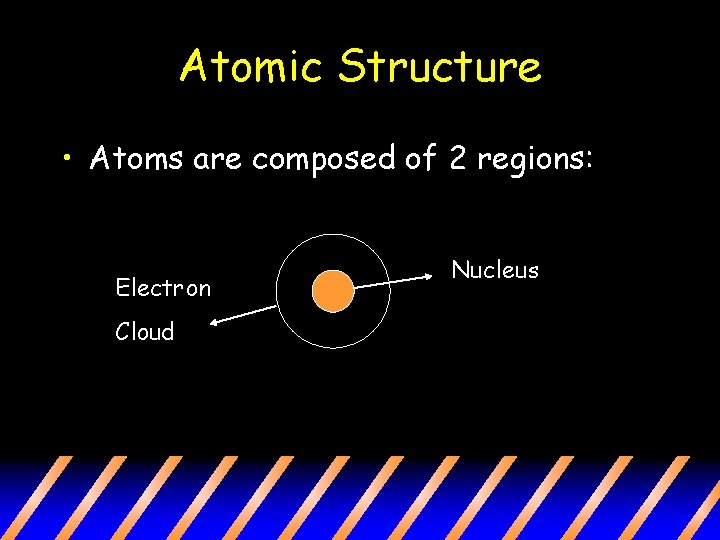
Atomic Structure • Atoms are composed of 2 regions: Electron Cloud Nucleus

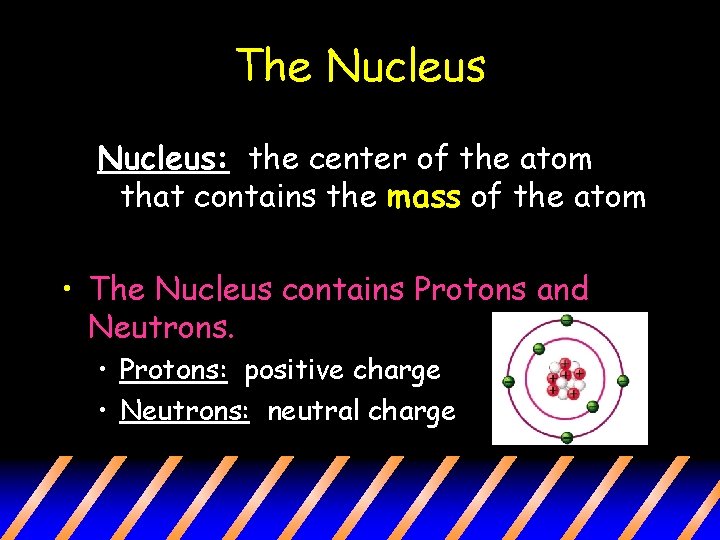
The Nucleus: the center of the atom that contains the mass of the atom • The Nucleus contains Protons and Neutrons. • Protons: positive charge • Neutrons: neutral charge

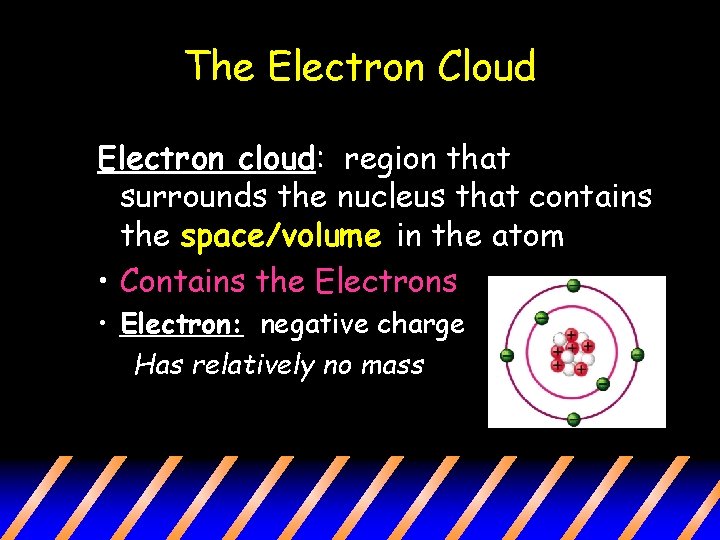
The Electron Cloud Electron cloud: region that surrounds the nucleus that contains the space/volume in the atom • Contains the Electrons • Electron: negative charge Has relatively no mass

An atom must be balanced! • In an atom: • The # of protons = the # of electrons • The neutrons have no charge; therefore they do not have to equal the number of protons or electrons

How can we find the number of protons, neutrons, and electrons? • Atomic number: this number indicates the number of protons in an atom • Atomic Mass: the number of protons and neutrons in the atom.

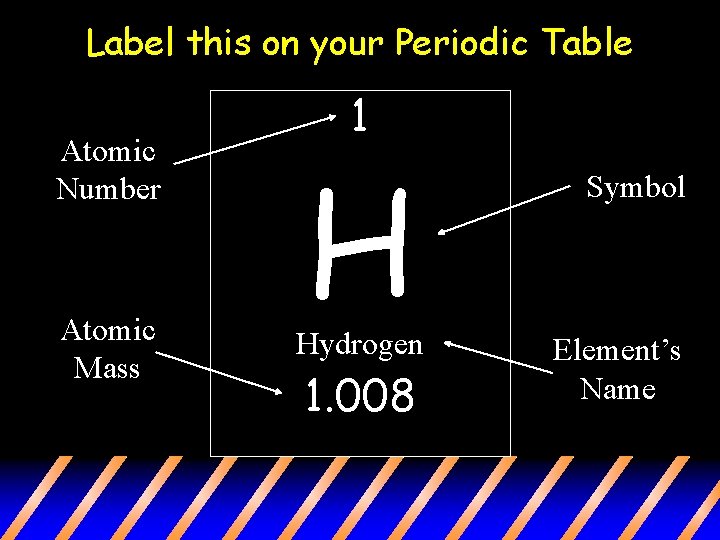
Label this on your Periodic Table Atomic Number Atomic Mass 1 H Hydrogen 1. 008 Symbol Element’s Name

Calculating the neutrons How can we find the Neutrons? # of neutrons = mass # - atomic #

What about the electrons? • The electrons are equal to the number of protons • Atomic # = protons = electrons

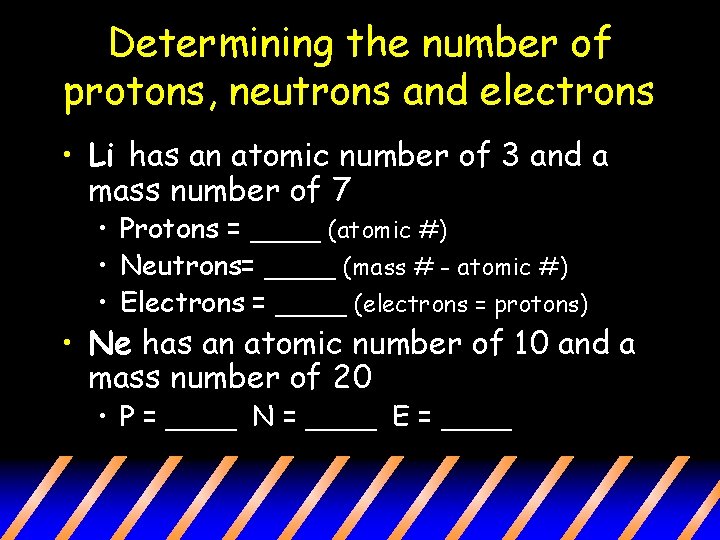
Determining the number of protons, neutrons and electrons • Li has an atomic number of 3 and a mass number of 7 • Protons = ____ (atomic #) • Neutrons= ____ (mass # - atomic #) • Electrons = ____ (electrons = protons) • Ne has an atomic number of 10 and a mass number of 20 • P = ____ N = ____ E = ____

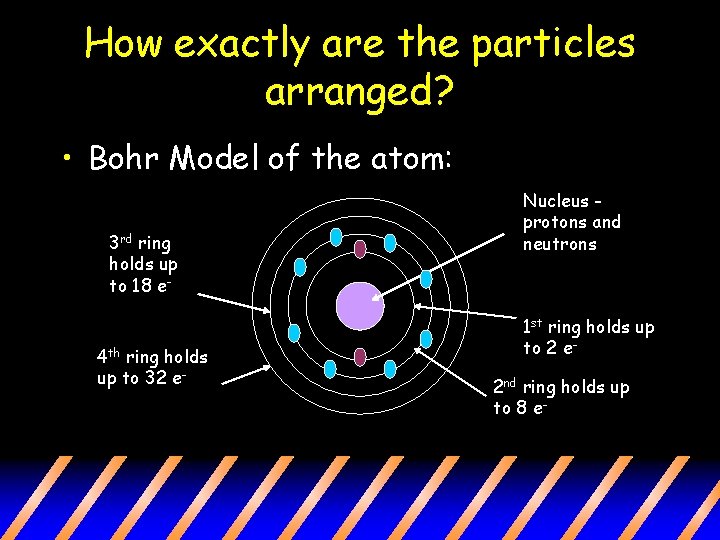
How exactly are the particles arranged? • Bohr Model of the atom: 3 rd ring holds up to 18 e- 4 th ring holds up to 32 e- Nucleus protons and neutrons 1 st ring holds up to 2 e 2 nd ring holds up to 8 e-

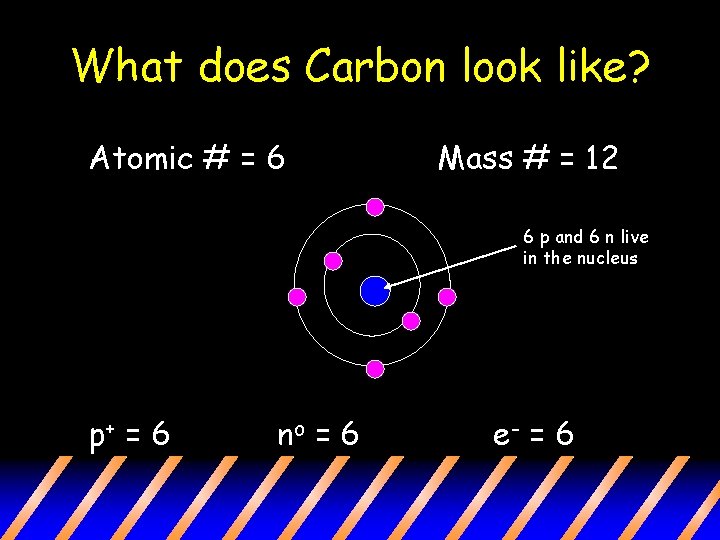
What does Carbon look like? Atomic # = 6 Mass # = 12 6 p and 6 n live in the nucleus p+ = 6 no = 6 e- = 6

Bohr Models • At the bottom of your notes, draw the Bohr Models for: • Lithium (Li) • Magnesium (Mg)

Atomic Structure and Bohr Model Practice On the front… • Boron (B) • Nitrogen (N) • Calcium (Ca) • Nickel (Ni) On the back… • Sodium (Na) • Beryllium (Be) • Neon (Ne) • Sulfur (S)

Bohr Model Trading Cards • Choose 2 elements from the Periodic Table. • Write the info (atomic #, symbol, etc. ) for those elements on the 2 papers provided. • Trade with 2 different people and draw the Bohr Model for that element on the back. • Write your name on the Bohr Model side!