Atomic Layer Deposition of Zirconium Oxide for Fuel

- Slides: 16

Atomic Layer Deposition of Zirconium Oxide for Fuel Cell Applications UIC REU – Summer 2011 AMRe. L Lab, UIC Department of Bioengineering and Department of Chemical Engineering Christine James University of Michigan, Department of Chemical Engineering

Overview • • Background Atomic Layer Deposition Data Collected Future Work

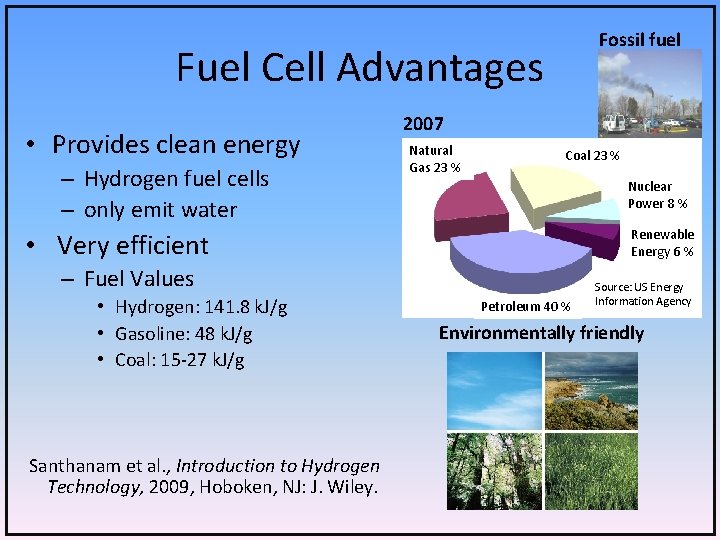
Fossil fuel Fuel Cell Advantages • Provides clean energy – Hydrogen fuel cells – only emit water 2007 Natural Gas 23 % Coal 23 % Nuclear Power 8 % Renewable Energy 6 % • Very efficient – Fuel Values • Hydrogen: 141. 8 k. J/g • Gasoline: 48 k. J/g • Coal: 15 -27 k. J/g Santhanam et al. , Introduction to Hydrogen Technology, 2009, Hoboken, NJ: J. Wiley. Petroleum 40 % Source: US Energy Information Agency Environmentally friendly

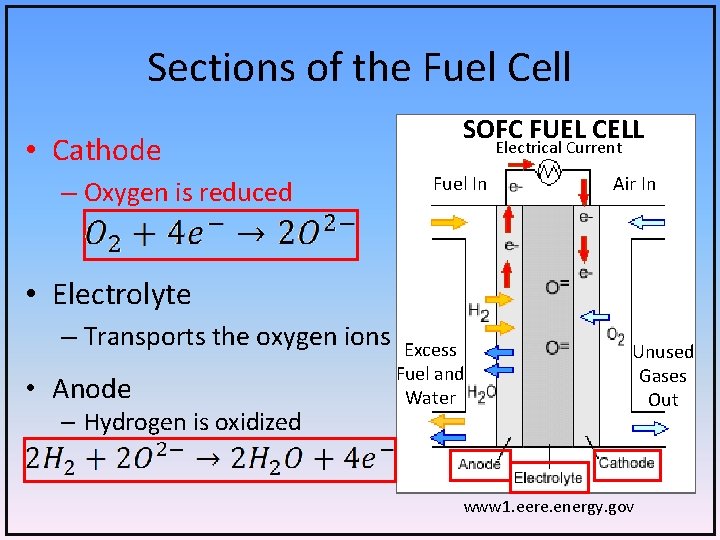
Sections of the Fuel Cell • Cathode – Oxygen is reduced SOFC FUEL CELL Electrical Current Fuel In Air In • Electrolyte – Transports the oxygen ions • Anode – Hydrogen is oxidized Excess Fuel and Water Unused Gases Out www 1. eere. energy. gov

Solid Oxide Fuel Cells (SOFCs) • Current SOFCs are high temperature – Temperature: about 1000 °C • Intermediate Temperature Fuel Cells – Temperature: 600 -800°C – Smaller scale applications – Allows use of alternate materials – Starts and stops faster – Reduces corrosion – Offers a wide range of possibilities

Problem with Reducing Temperature • High temperatures needed to transport O 2 - ions – Requirement can be as high as 1200° C – Low temperatures cause ionic resistance Approach • Deposit electrolytes and analyze – Samples from atomic to bulk-like thickness – Method to be used: • Atomic Layer Deposition • Deposit oxide layers on silicon then platinum (Pt)

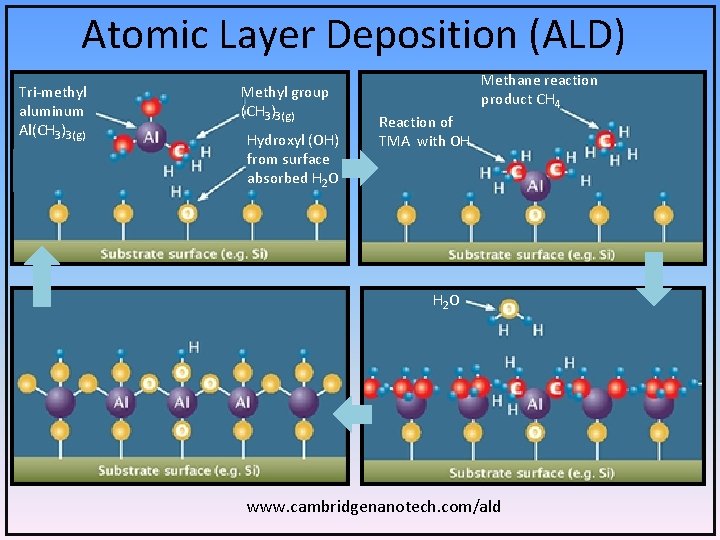
Atomic Layer Deposition (ALD) Tri-methyl aluminum Al(CH 3)3(g) Methyl group (CH 3)3(g) Hydroxyl (OH) from surface absorbed H 2 O Reaction of TMA with OH Methane reaction product CH 4 H 2 O www. cambridgenanotech. com/ald

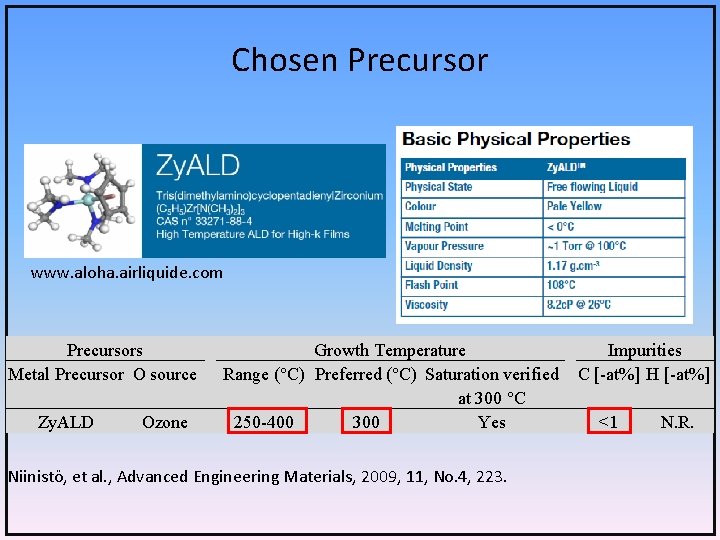
Chosen Precursor www. aloha. airliquide. com Precursors Metal Precursor O source Zy. ALD Ozone Growth Temperature Impurities Range (°C) Preferred (°C) Saturation verified C [-at%] H [-at%] at 300 °C 250 -400 300 Yes <1 N. R. Niinistö, et al. , Advanced Engineering Materials, 2009, 11, No. 4, 223.

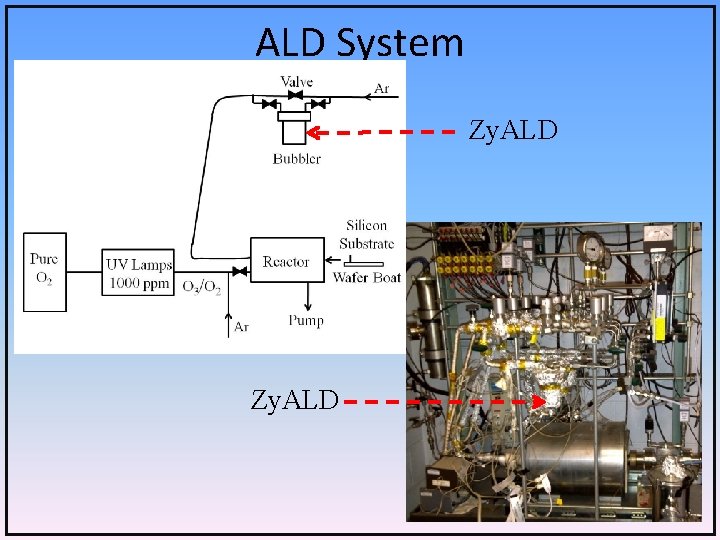
ALD System Zy. ALD

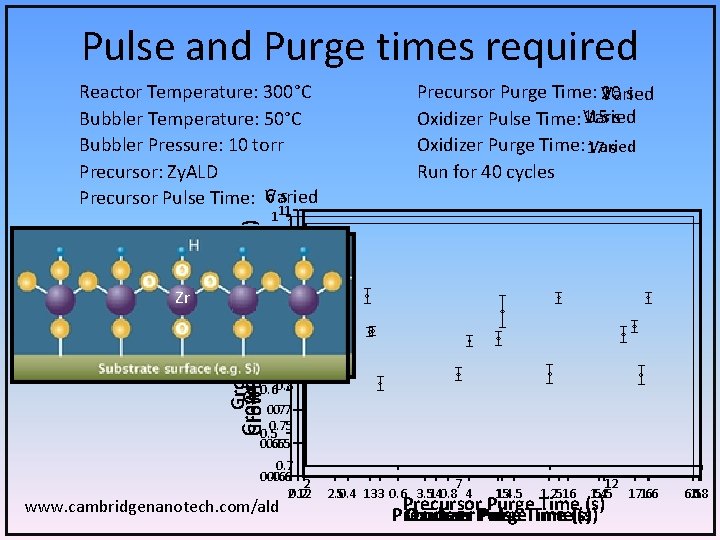
Pulse and Purge times required Reactor Temperature: 300°C Bubbler Temperature: 50°C Bubbler Pressure: 10 torr Precursor: Zy. ALD 6 s Precursor Pulse Time: Varied Precursor Purge Time: 10 20 s Varied 1 ss 1. 5 Oxidizer Pulse Time: Varied Oxidizer Purge Time: 17 Varied s Run for 40 cycles Zr Growth Rate (Å/cycle) Growth Rate (Å/cycle) 111 1 0. 95 0. 9 0. 80. 9 0. 85 0. 75 0. 60. 8 0. 75 0. 65 0. 7 0. 4 0. 6 2 20. 2 12 www. cambridgenanotech. com/ald 7 2. 50. 4 133 0. 6 3. 5140. 8 4 1154. 5 1. 2516 12 1. 4 5. 5 171. 6 6 Precursor Purge Time (s) Precursor Oxidizer Pulse Purge. Time(s) 6. 5 1. 8 18

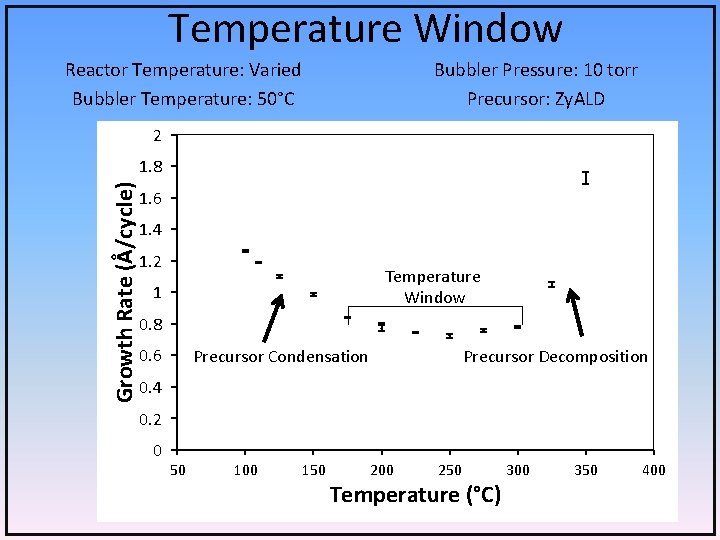
Temperature Window Reactor Temperature: Varied Bubbler Temperature: 50°C Bubbler Pressure: 10 torr Precursor: Zy. ALD 2 Growth Rate (Å/cycle) 1. 8 1. 6 1. 4 1. 2 Temperature Window 1 0. 8 0. 6 Precursor Condensation Precursor Decomposition 0. 4 0. 2 0 50 100 150 200 250 Temperature (°C) 300 350 400

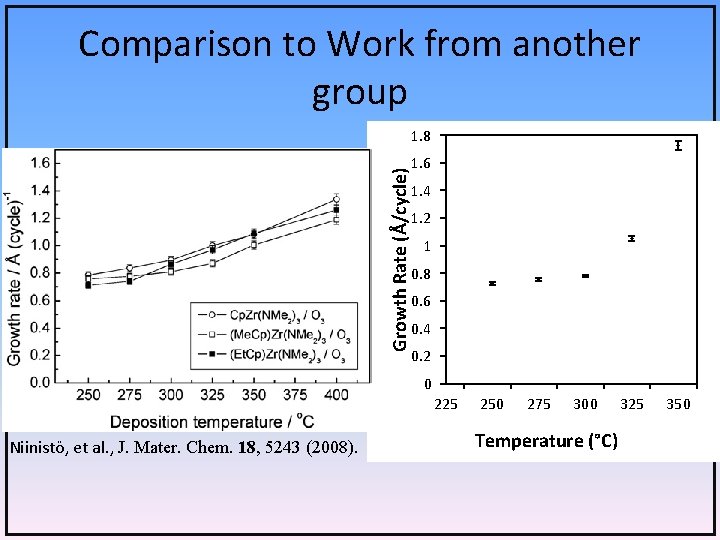
Comparison to Work from another group Growth Rate (Å/cycle) 1. 8 1. 6 1. 4 1. 2 1 0. 8 0. 6 0. 4 0. 2 0 225 Niinistö, et al. , J. Mater. Chem. 18, 5243 (2008). 250 275 300 Temperature (°C) 325 350

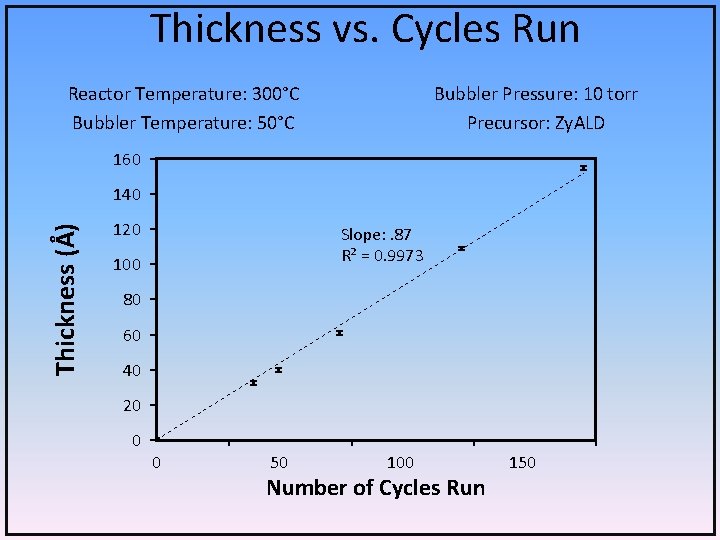
Thickness vs. Cycles Run Reactor Temperature: 300°C Bubbler Temperature: 50°C Bubbler Pressure: 10 torr Precursor: Zy. ALD 160 Thickness (Å) 140 120 Slope: . 87 R² = 0. 9973 100 80 60 40 20 0 0 50 100 Number of Cycles Run 150

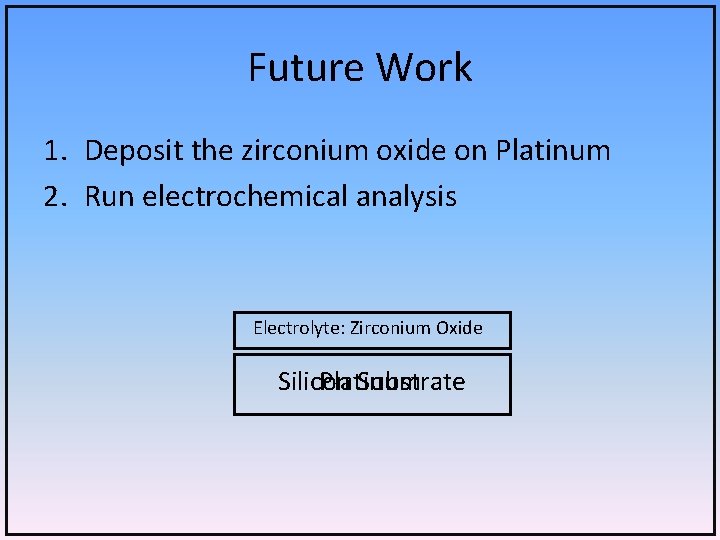
Future Work 1. Deposit the zirconium oxide on Platinum 2. Run electrochemical analysis Electrolyte: Zirconium Oxide Silicon Platinum Substrate

Summary • Goal is to lower operating temperature of the fuel cell – By decreasing electrolyte layer thickness • Atomic Layer Deposition (ALD) is being used • Have determined some necessary parameters: – Pulse and Purge times – Temperature Window for ALD • Have compared cycles and thickness – Proved linear relationship • Next Steps: – Deposit on Platinum – Run Electrochemical analysis

Acknowledgements • National Science Foundation – EEC-NSF Grant # 1062943 • Graduate Mentor: Runshen Xu • Professor Takoudis and Professor Jursich
 Atomic layer deposition wiki
Atomic layer deposition wiki Atomic layer deposition
Atomic layer deposition Atomic layer deposition for semiconductors
Atomic layer deposition for semiconductors Basic oxides
Basic oxides Solid oxide fuel cell
Solid oxide fuel cell Zirconium procurement
Zirconium procurement Radius of lithium
Radius of lithium How do you calculate atomic mass
How do you calculate atomic mass Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Distinguish between mass number and atomic mass.
Distinguish between mass number and atomic mass. Periodic trends in the modern periodic table
Periodic trends in the modern periodic table Atomic number vs atomic radius
Atomic number vs atomic radius Atomic layer etching
Atomic layer etching Layer 2 e layer 3
Layer 2 e layer 3 Secure socket layer and transport layer security
Secure socket layer and transport layer security Layer-by-layer assembly
Layer-by-layer assembly Fig 19
Fig 19