Aquatic Ecology Freshwater Part 4 Prof Dr N


















































- Slides: 75

Aquatic Ecology Freshwater - Part 4 Prof. Dr. N. De Pauw Laboratory of Environmental Toxicology and Aquatic Ecology

Course Contents 1. Place of limnology in natural sciences 2. Historical development of limnology 3. The water cycle, distribution, age and genesis of inland waters 4. Structure and physical properties of water 5. Physical relationships in natural water bodies 6. Communities of living organisms in natural waters 7. Materials budget in natural waters I (= gases, solid and dissolved substances, importance of sediments) 8. Materials budget in natural waters II (= production, consumption, decomposition)

7. Materials budget of natural waters I Contents (1) 7. 1. Introduction 7. 2. Dissolved gases and dissolved solids 7. 3. Gases dissolved in water 7. 3. 1. Solubility of gases in water 7. 3. 2. Oxygen content and oxygen budget 7. 3. 3. Carbon dioxide, carbonic acid and carbonates 7. 3. 4. Methane and hydrogen sulphide 7. 3. 5. Nitrogen

7. Materials budget of natural waters I Contents (2) 7. 4 Solids dissolved in water 7. 4. 1. Solubility of solids in water 7. 4. 2. Nitrogen compounds 7. 4. 3. Phosphorous compounds 7. 4. 4. Sulphur compounds 7. 4. 5. Iron and manganese 7. 4. 6. Silica 7. 5. Dissolved organic matter in natural waters 7. 6. Sediment and the materials budget 7. 7. Materials budget of flowing waters

7. Materials budget of natural waters I 7. 1. Introduction = Sum of materials and energy turnover in an ecosystem FOUNDATIONS 1. Water as a solvent 2. Dissolved and particulate materials 3. Organisms in water

7. Materials budget of natural waters I 7. 1. Introduction Characterized by the following processes 1. Bio-activity of organisms • Production • Consumption • Organisms in water 2. Chemical and biological transport of material + energy • Into the sediment • Release from the sediment 3. Transport of material + energy • In lakes : seasonal rhythm • In rivers : unidirectional transport 4. Exchange • With atmosphere (precipitation) • In and outflow • Absorption and desorption (suspended particles)


7. Materials budget of natural waters I Contents (1) 7. 1. Introduction 7. 2. Dissolved gases and dissolved solids 7. 3. Gases dissolved in water 7. 3. 1. Solubility of gases in water 7. 3. 2. Oxygen content and oxygen budget 7. 3. 3. Carbon dioxide, carbonic acid and carbonates 7. 3. 4. Methane and hydrogen sulphide 7. 3. 5. Nitrogen

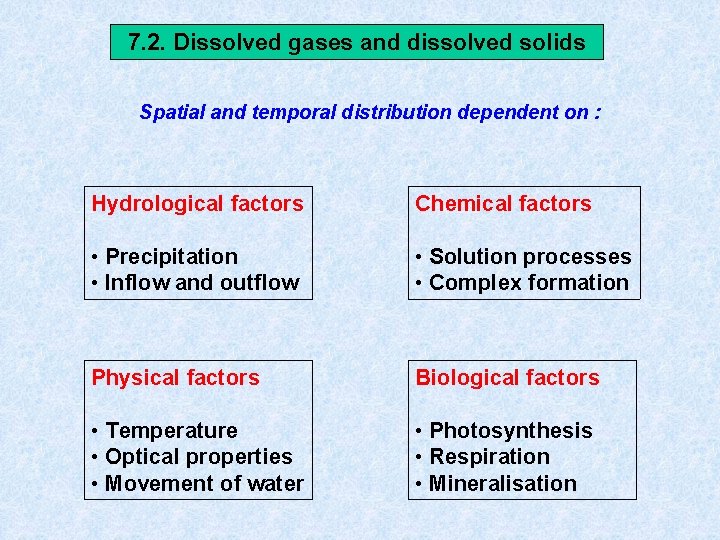
7. 2. Dissolved gases and dissolved solids Spatial and temporal distribution dependent on : Hydrological factors Chemical factors • Precipitation • Inflow and outflow • Solution processes • Complex formation Physical factors Biological factors • Temperature • Optical properties • Movement of water • Photosynthesis • Respiration • Mineralisation

7. 2. Dissolved gases and solids Physico-chemical processes • Dissolution and precipitation of solids • Absorption and desorption of gases • Ion exchange at solid surfaces Chemical processes • Redox processes • Soluble complex formation • Hydrolytic cleavage Biochemical processes • Mineralisation of organic matter • Photosynthesis • Respiration


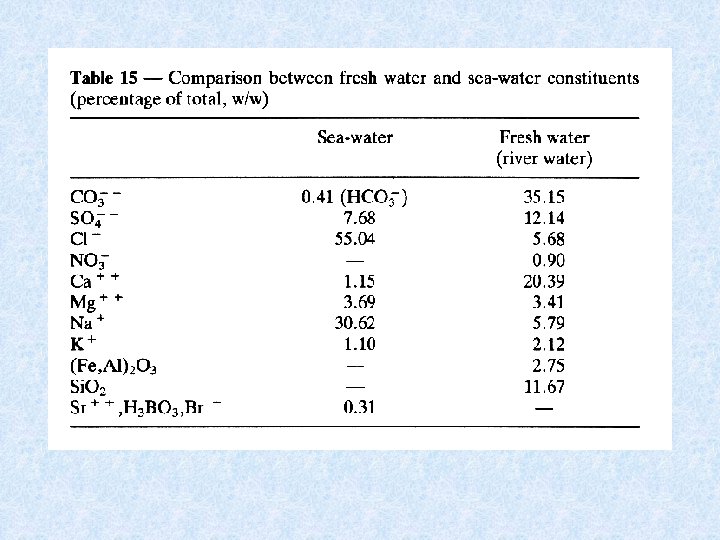
Dissolved substances in fresh and seawater In freshwater : calcium carbonate + silicates + nitrates In seawater : sodium chloride Besides inorganic materials indefinite number of organic compounds LAW OF THE MINIMUM (Liebig): Yield dependent on whatever growth factor is at a minimum in proportion to all similar factors (e. g. phosphorous vs nitrogen)



7. Materials budget of natural waters I Contents (1) 7. 1. Introduction 7. 2. Dissolved gases and dissolved solids 7. 3. Gases dissolved in water 7. 3. 1. Solubility of gasses in water 7. 3. 2. Oxygen content and oxygen budget 7. 3. 3. Carbon dioxide, carbonic acid and carbonates 7. 3. 4. Methane and hydrogen sulphide 7. 3. 5. Nitrogen

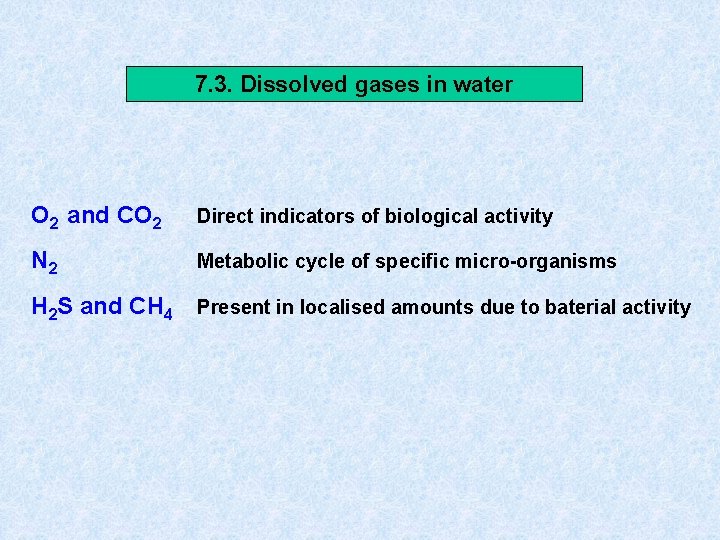
7. 3. Dissolved gases in water O 2 and CO 2 Direct indicators of biological activity N 2 Metabolic cycle of specific micro-organisms H 2 S and CH 4 Present in localised amounts due to baterial activity

7. 3. 1. Solubility of gases in water Henry’s law: Solubility of a gas decreases with : • Increasing temperature • Decreasing pressure Quantity of dissolved gas : Cs = Ks * P t Cs = Saturation concentration of the gas Ks = Temperature dependent solubility Pt = Partial pressure of the gas CO 2 has highest solubility CO 2 + H 2 O H 2 CO 3 / Ca. CO 3



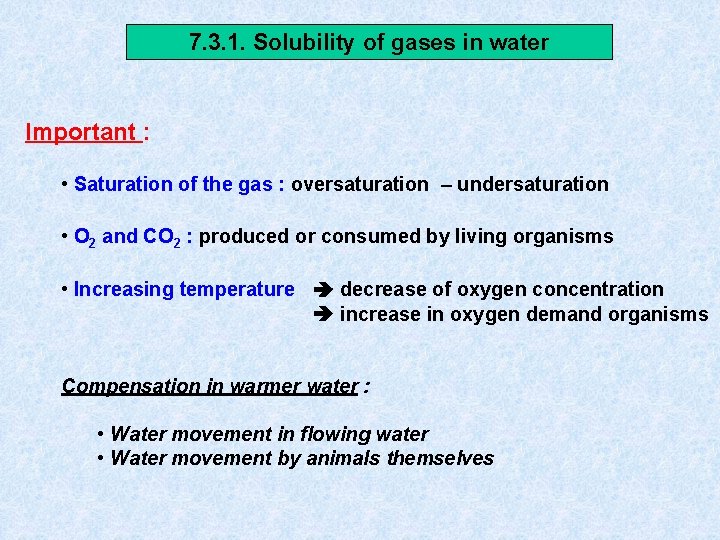
7. 3. 1. Solubility of gases in water Important : • Saturation of the gas : oversaturation – undersaturation • O 2 and CO 2 : produced or consumed by living organisms • Increasing temperature decrease of oxygen concentration increase in oxygen demand organisms Compensation in warmer water : • Water movement in flowing water • Water movement by animals themselves


7. 3. 2. Oxygen content and oxygen budget of surface waters Factors affecting the oxygen balance INPUT 1. From atmosphere 2. Photosynthesis LOSSES 1. Respiration 2. Decomposition mineralisation 3. Losses to atmosphere Oxygen balance less positive if : • Input decreases • Losses increase Deductions : 1. Flowing waters with rapid movements and shallower depth have a more favourable oxygen balance than still waters 2. Input of organic matter into water body has an adverse effect on its oxygen balance (greater effect in still than in flowing water)

Dissolved oxygen in lakes O 2 from atmosphere water greater depths by water movements: During seasonal turnover : O 2 rich water bottom During summer stagnation phase : • In epilimnion: • O 2 from atmosphere + photosynthesis • O 2 oversaturation during the day + O 2 deficit during the night • Diurnal fluctuations of p. H and CO 2 • In hypolimnion: • Exclusively oxygen depletion processes : Heaviest oxygen demand imposed by microbial mineralisation of plant and animal residues deposited in profundal zone • Quantity of organic matter dependent on : • Production in epilimnion • Sinking and degradation rate of dead organisms • Depth of the water

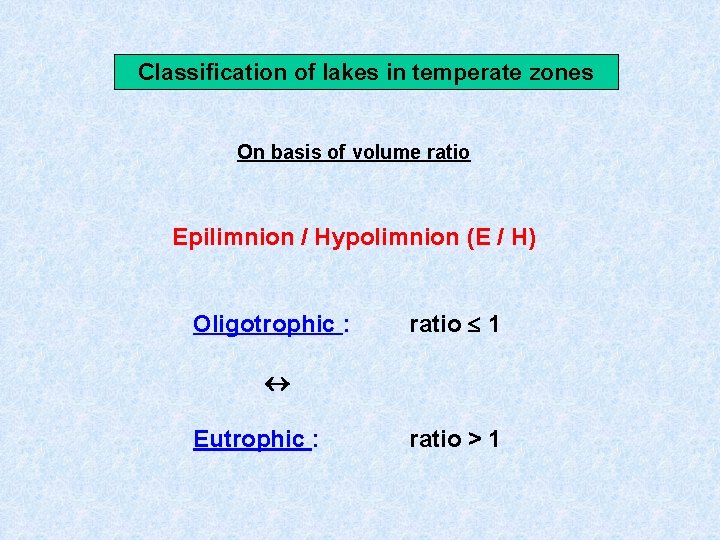
Classification of lakes in temperate zones On basis of volume ratio Epilimnion / Hypolimnion (E / H) Oligotrophic : ratio 1 Eutrophic : ratio > 1


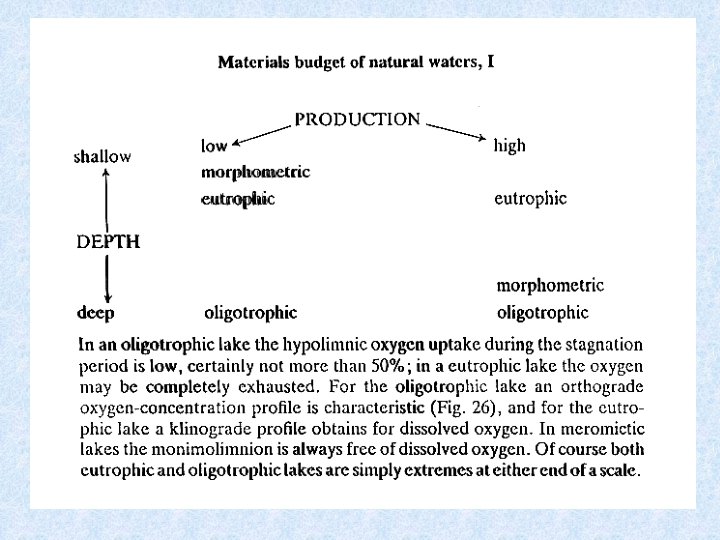
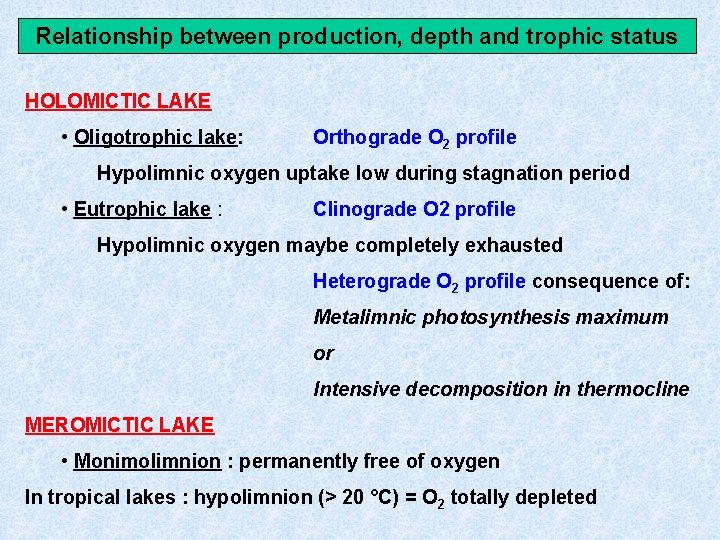
Relationship between production, depth and trophic status HOLOMICTIC LAKE • Oligotrophic lake: Orthograde O 2 profile Hypolimnic oxygen uptake low during stagnation period • Eutrophic lake : Clinograde O 2 profile Hypolimnic oxygen maybe completely exhausted Heterograde O 2 profile consequence of: Metalimnic photosynthesis maximum or Intensive decomposition in thermocline MEROMICTIC LAKE • Monimolimnion : permanently free of oxygen In tropical lakes : hypolimnion (> 20 °C) = O 2 totally depleted




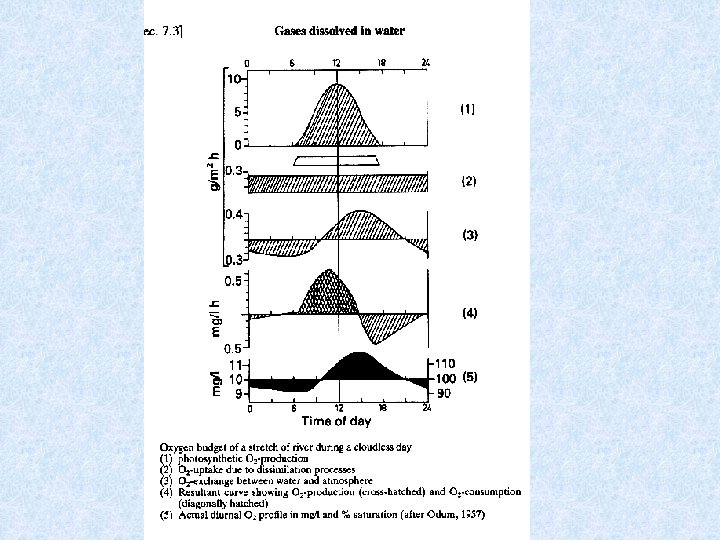
Oxygen budget of flowing waters Oxygen budget affected by : • Degradable organic matter carried along • Organic effluents Clues provided to oxygen budget : • In lakes : Vertical differences in O 2 concentration • In rivers : Diurnal O 2 saturation profile

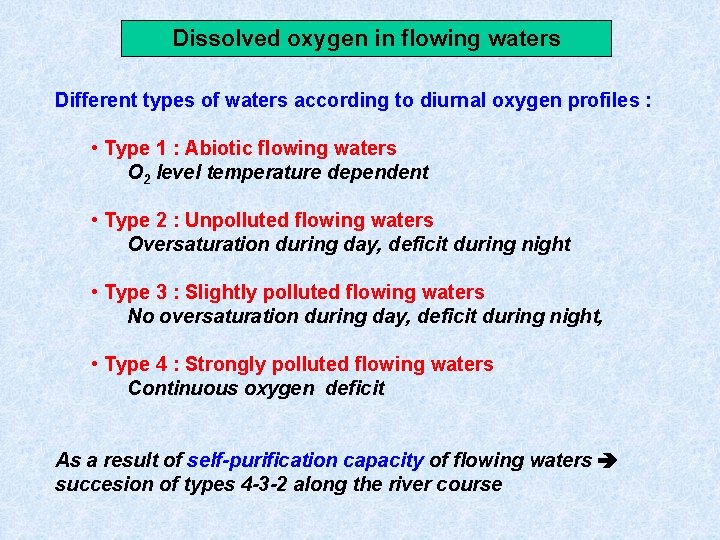
Dissolved oxygen in flowing waters Different types of waters according to diurnal oxygen profiles : • Type 1 : Abiotic flowing waters O 2 level temperature dependent • Type 2 : Unpolluted flowing waters Oversaturation during day, deficit during night • Type 3 : Slightly polluted flowing waters No oversaturation during day, deficit during night, • Type 4 : Strongly polluted flowing waters Continuous oxygen deficit As a result of self-purification capacity of flowing waters succesion of types 4 -3 -2 along the river course


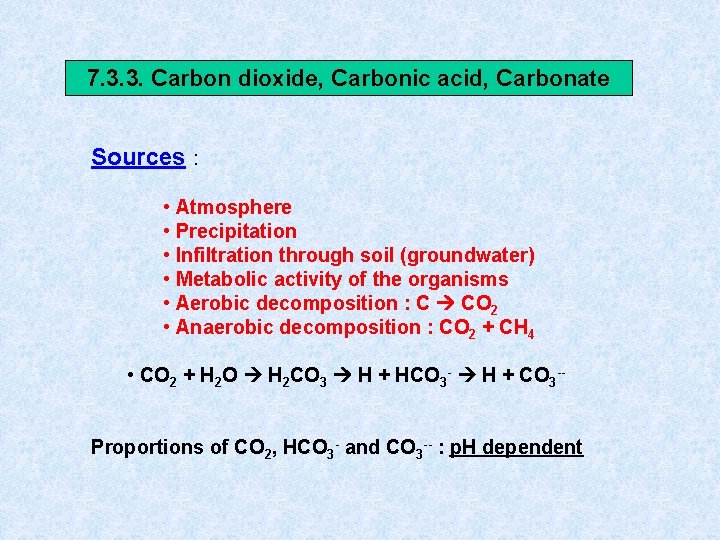
7. 3. 3. Carbon dioxide, Carbonic acid, Carbonate Sources : • Atmosphere • Precipitation • Infiltration through soil (groundwater) • Metabolic activity of the organisms • Aerobic decomposition : C CO 2 • Anaerobic decomposition : CO 2 + CH 4 • CO 2 + H 2 O H 2 CO 3 H + HCO 3 - H + CO 3 -Proportions of CO 2, HCO 3 - and CO 3 -- : p. H dependent



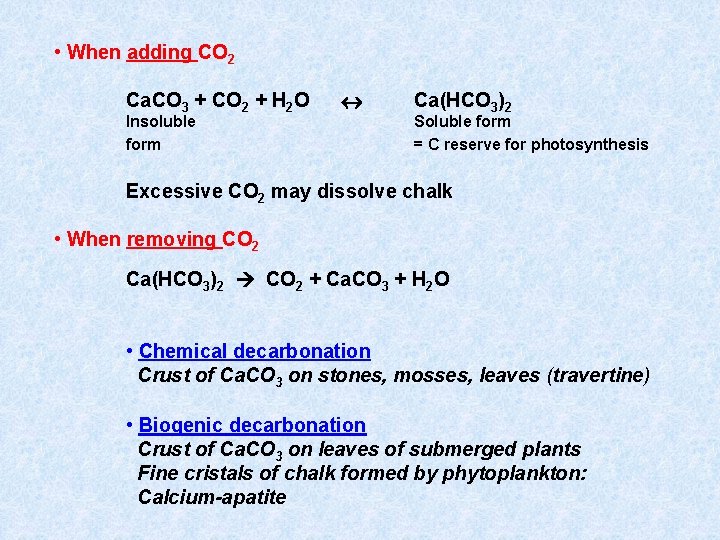
• When adding CO 2 Ca. CO 3 + CO 2 + H 2 O Insoluble form Ca(HCO 3)2 Soluble form = C reserve for photosynthesis Excessive CO 2 may dissolve chalk • When removing CO 2 Ca(HCO 3)2 CO 2 + Ca. CO 3 + H 2 O • Chemical decarbonation Crust of Ca. CO 3 on stones, mosses, leaves (travertine) • Biogenic decarbonation Crust of Ca. CO 3 on leaves of submerged plants Fine cristals of chalk formed by phytoplankton: Calcium-apatite

By the presence of calcium carbonate in its blue-green water, the Havasu creek in the Grand Canyon National park, slowly deposits stone called travertine.

Tuff formations at Mono Lake (California). They were formed by the interaction of calcareous groundwater with the Ca. CO 3 and other minerals in the lake.

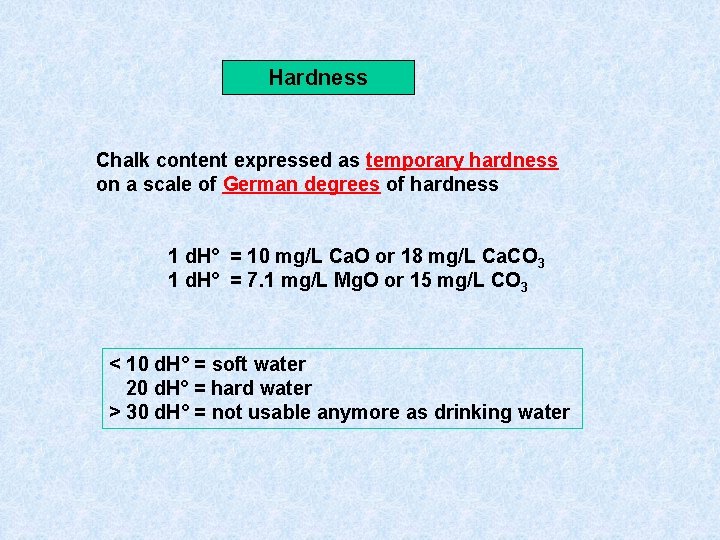
Hardness Chalk content expressed as temporary hardness on a scale of German degrees of hardness 1 d. H° = 10 mg/L Ca. O or 18 mg/L Ca. CO 3 1 d. H° = 7. 1 mg/L Mg. O or 15 mg/L CO 3 < 10 d. H° = soft water 20 d. H° = hard water > 30 d. H° = not usable anymore as drinking water

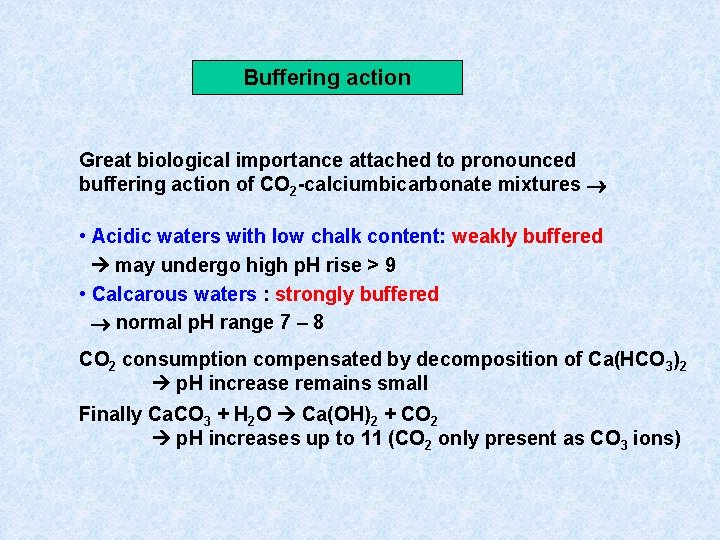
Buffering action Great biological importance attached to pronounced buffering action of CO 2 -calciumbicarbonate mixtures • Acidic waters with low chalk content: weakly buffered may undergo high p. H rise > 9 • Calcarous waters : strongly buffered normal p. H range 7 – 8 CO 2 consumption compensated by decomposition of Ca(HCO 3)2 p. H increase remains small Finally Ca. CO 3 + H 2 O Ca(OH)2 + CO 2 p. H increases up to 11 (CO 2 only present as CO 3 ions)

Abatement of acidification by means of addition of chalk

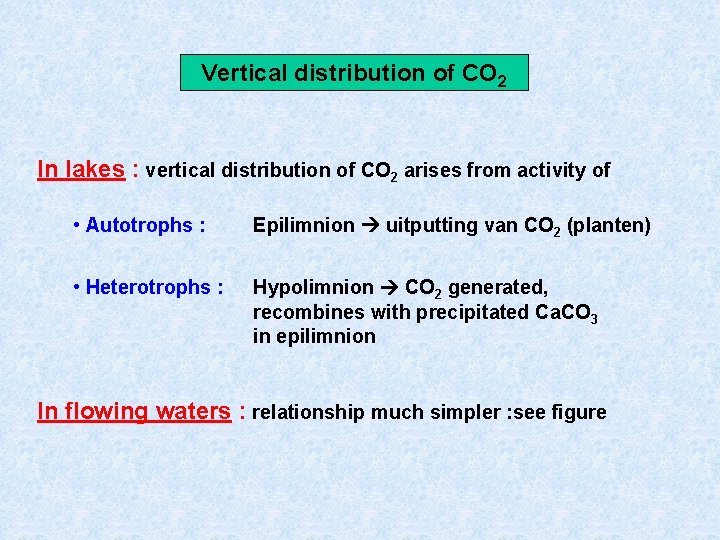
Vertical distribution of CO 2 In lakes : vertical distribution of CO 2 arises from activity of • Autotrophs : Epilimnion uitputting van CO 2 (planten) • Heterotrophs : Hypolimnion CO 2 generated, recombines with precipitated Ca. CO 3 in epilimnion In flowing waters : relationship much simpler : see figure


7. 3. 4. Methane and hydrogen sulphide Result of anaerobic decomposition of organic matter CH 4 Released to atmosphere Oxidized to formaldehyde H 2 S Dissolves readily in water N 2 Certain bacteria (cyanobacteria) can fix N N 2 + 12 ATP + 6 H 2 NH 3 + 12 ADP + 12 P N-fixation at sediment-water interface


7. Materials budget of natural waters I Contents (2) 7. 4 Solids dissolved in water 7. 4. 1. Solubility of solids in water 7. 4. 2. Nitrogen compounds 7. 4. 3. Phosphorous compounds 7. 4. 4. Sulphur compounds 7. 4. 5. Iron and manganese 7. 4. 6. Silica 7. 5. Dissolved organic matter in natural waters 7. 6. Sediment and the materials budget 7. 7. Materials budget of flowing waters

7. 4. 1. Solubility of solids in water • Water is a particularly suitable solvent for electrolytes: - High dielectric constant - Ability to form hydrates • Solubility of solid substances dependent on: - p. H - Eh • Most substances dissolve either : - In molecular form - As ion - In colloidal form




7. 4. 2. Nitrogen compounds Nitrogen occurs in the form of numerous compounds: Inorganic form • NO 3, NO 2, NH 4 Organic form • Intermediate stages of microbial protein decomposition ; Excretion products • Amino-acids, Enzymes NO 3 and NH 4 = nitrogen sources for photo-autotrophic plants NH 4 = result of decomposition of organic residues

7. 4. 2. Nitrogen compounds Important In lakes • N 2 binding : Blue-green algae, Azotobacter, Clostridium • N-assimilation : N 2, NH 4, NO 3 organic nitrogen • Ammonification: organic N NH 4 • Nitrate reduction : NO 3 NH 4 • Nitrification : NH 4 NO 2 NO 3 (Nitrosomonas & Nitrobacter) • Denitrification : NO 3 N 2 (Pseudobacter) In flowing waters • Not polluted : NO 3 most important N-component • Polluted : NH 4 gradually oxidized to NO 3



7. 4. 3. Phosphorous compounds • P often only as traces • P often growth limiting factor Eutrophication involves primarily increase in PO 4 levels. Different fractions : • Dissolved inorganic phosphate = orthofosphate + polyphosphates • Dissolved organic phosphate • Particular organic phosphate = organisms and detritus

7. 4. 3. Phosphorous compounds In trophogenic zone : • Dissolved Inorganic phosphate taken up by photo-autotrophic producers organic compounds of food chain • Major part released again into epilimnion • Lesser part sediments (adsorption, precipitated as Fe. PO 4) > 10 % O 2 : release of PO 4 in water



7. 4. 4. Sulphur compounds Inorganic sulphur components in water : SO 4 (sulphate) Of great importance: Activity of micro-organisms in sulphur cycle (chemo+photoautotrophic production) • Desulfuricans organisms reduce SO 4 tot H 2 S + sulfiden (sediments) C 6 H 12 O 6 + 3 K 2 SO 4 6 KHCO 3 + 3 H 2 S Microbial decomposition of proteins H 2 S = Facultative chemo-autotrophic anaerobic sulphur bacteria • Sulfuricans organisms oxidize H 2 S S SO 4 2 H 2 S + O 2 2 H 2 O + 2 S 5 S + 6 KNO 3 + 2 H 2 O 2 N 2 + 3 K 2 SO 4 + 2 H 2 SO 4 = Chemo-autotrophic colourless aerobic sulphur bacteria + thiobacteria = Photo-autotrophic coloured anaerobic sulphur bacteria



7. 4. 5. Iron present in natural waters only in small amounts Exception : groundwater may contain large quantities of : • Dissolved iron = bivalent iron (as Fe(HCO 3)2) • Insoluble iron = trivalent iron (as Fe(OH)3) Bivalent iron remains in solution if : • O 2 < 50 % • presence of degradable organic matter • >> free CO 2 • p. H < 7. 5 Fe(HCO 3)2 + O 2 precipitation of Fe(OH)3 + Fe. O(OH)

7. 4. 5. Iron • Iron bacteria (Thiobacillus) are involved in process of Fe- precipitation: oxidize Fe 2+ Fe 3+ (chemo litho authotrophic bacteria ). • Fe remains in solution in the hypolimnion of eutrophic lakes during stagnation period • In trophogenic zone (epilimnion) small amounts of dissolved iron quickly used up by producers 7. 4. 5. Manganese May be released from sediments when O 2 still several mg/L.

7. 4. 6. Silica (silicic acid) Dissolved silica = Building material for diatoms Dissolution of silica from the sediments : Takes place between interstitial water and free water Affected by : • Temperature • Age of sediments of biogenic origin • p. H • Bottom dwelling animals

7. Materials budget of natural waters I Contents (2) 7. 4 Solids dissolved in water 7. 4. 1. Solubility of solids in water 7. 4. 2. Nitrogen compounds 7. 4. 3. Phosphorous compounds 7. 4. 4. Sulphur compounds 7. 4. 5. Iron and manganese 7. 4. 6. Silica 7. 5. Dissolved organic matter in natural waters 7. 6. Sediment and the materials budget 7. 7. Materials budget of flowing waters

7. 5. Dissolved organic matter in natural waters Dissolved organic matter >> particulate organic matter DOM >> POM Origin of DOM : • Losses due to photorespiration • Secretion of products of photosynthesis (algae + plants) • Excretions by bacteria • Hydrolysis + decomposition of dead organisms Important group = HUMIC SUBSTANCES (humic acids + fulvic acids) Origin : • Incomplete breakdown of plant residues in water bodies • Affect the materials budget: complex formation with heavy metals • Prevents precipitation - ensure availability to primary producers

7. Materials budget of natural waters I Contents (2) 7. 4 Solids dissolved in water 7. 4. 1. Solubility of solids in water 7. 4. 2. Nitrogen compounds 7. 4. 3. Phosphorous compounds 7. 4. 4. Sulphur compounds 7. 4. 5. Iron and manganese 7. 4. 6. Silica 7. 5. Dissolved organic matter in natural waters 7. 6. Sediment and the materials budget 7. 7. Materials budget of flowing waters

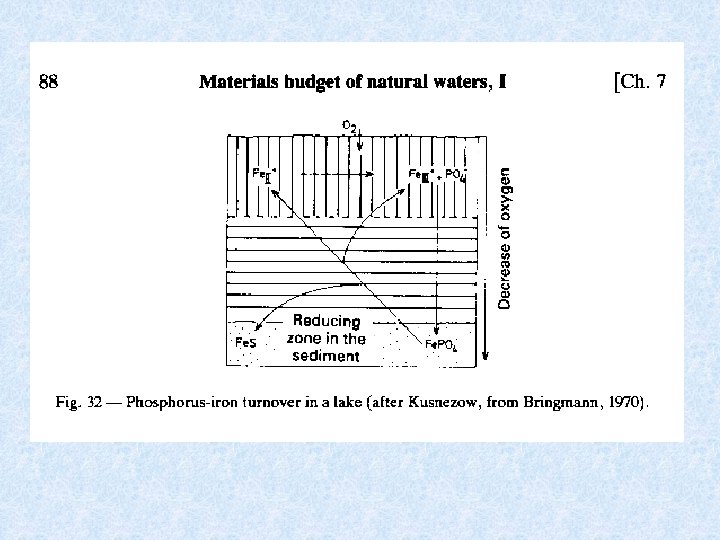
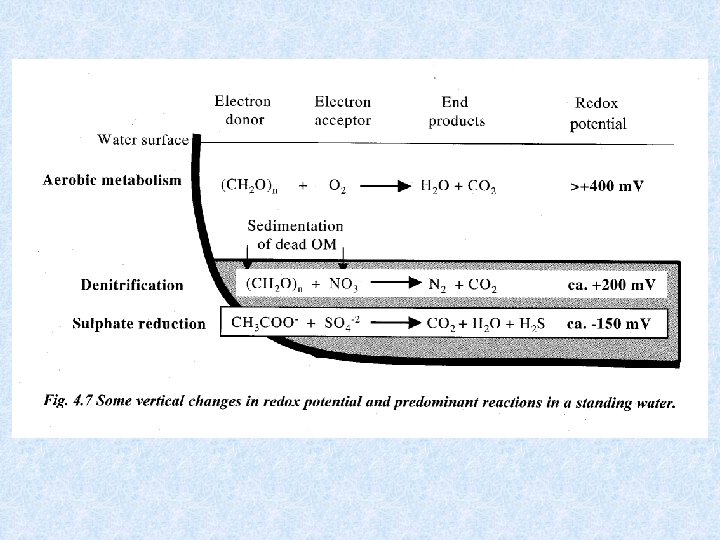
7. 6. Sediment and the materials budget Important interactions between water and sediment In contact zone of sediment surface : • Precipitation • Dissolution • Exchange processes : • Absorption or release • Determining factor = redoxpotentiaal Inorganic phosphate : shift of Fe + P from : Anaerobic conditions in deeper sediments sediment surface release of P in water at sediment surface Organically bound P in sediment: stable fraction

7. 6. Sediment and the materials budget Redox potential in upper sediment layer (several cm) Reducing and oxidizing conditions change with time as a consequence of periodic succession of turnover and stagnation phase and amount of decomposed organic matter • In oligotrophic water and during turnover in eutrophic lakes: high oxygen content in deep water : Eh = 0. 6 V • In eutrophic water during stagnation phase : low oxygen content in deep water: reducing zone migrates upward from deeper sediment to sediment-water contact zone : Eh decreases at Eh = 0. 2 V : Fe 2+ + PO 4 go into solution at Eh = 0. 1 – 0 V : reduction of SO 4 H 2 S + S




7. Materials budget of natural waters I Contents (2) 7. 4 Solids dissolved in water 7. 4. 1. Solubility of solids in water 7. 4. 2. Nitrogen compounds 7. 4. 3. Phosphorous compounds 7. 4. 4. Sulphur compounds 7. 4. 5. Iron and manganese 7. 4. 6. Silica 7. 5. Dissolved organic matter in natural waters 7. 6. Sediment and the materials budget 7. 7. Materials budget of flowing waters

7. 7. Materials budget of flowing water More dependent on ecological structure of catchment (= open system) Less dependent on internal metabolism (cf. lakes = closed system) Smaller rivers reflect geochemical situation of their catchments : Geochemical types : Bicarbonate type Catchment area : chalk and dolomite rocks Ca(HCO 3)2 – Mg(HCO 3)2 neutral – alkaline and well buffered Sulphate type Catchment area : gipsum deposits Ca. SO 4 Chloride type Catchment area : salt deposits or salination Na. Cl of Na. HCO 3 Silicate type Catchment area : silicate rocks low in lime, poor in electrolytes neutral – acid, weakly buffered

7. 7. Materials budget of flowing waters Larger rivers integrate the diverse structures Main factors controlling the chemistry of watercourse during its transit time : • Solution processes • Evaporation – precipitation • Adsorption – desorption on suspended solids and sediments • Internal reactions • Exchange with atmosphere Processes in flowing waters limited by relatively short transit time : average 10 days = major difference with stagnant waters (e. g. lakes) (not water movement !).
 Aquatic ecology definition
Aquatic ecology definition Ecology part 1
Ecology part 1 Community level ecology
Community level ecology Pie chart of freshwater and saltwater
Pie chart of freshwater and saltwater Description of freshwater
Description of freshwater Lentic ecosystem
Lentic ecosystem Saber toothed anchovy biome
Saber toothed anchovy biome Freshwater animals adaptations
Freshwater animals adaptations Freshwater fish marketing
Freshwater fish marketing Many freshwater invertebrates eliminate ammonia by
Many freshwater invertebrates eliminate ammonia by Freshwater senior campus
Freshwater senior campus Fun facts about freshwater ecosystems
Fun facts about freshwater ecosystems What are estuaries
What are estuaries Tertiary consumers in freshwater
Tertiary consumers in freshwater Desert living and nonliving things
Desert living and nonliving things Freshwater zones diagram
Freshwater zones diagram Estuary biome map
Estuary biome map Order of animal
Order of animal Freshwater biomes climate
Freshwater biomes climate Bibliography
Bibliography Wetland ecosystem definition
Wetland ecosystem definition Freshwater animal adaptations
Freshwater animal adaptations Marine reptiles
Marine reptiles 14.2 uses of freshwater
14.2 uses of freshwater Climate of freshwater wetlands
Climate of freshwater wetlands Abiotic factors in freshwater
Abiotic factors in freshwater Climate by latitude
Climate by latitude Upstate freshwater institute
Upstate freshwater institute Freshwater heterotrophs
Freshwater heterotrophs Freshwater
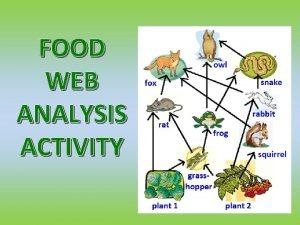
Freshwater Food web examples
Food web examples Freshwater basin
Freshwater basin Texas freshwater fish identification
Texas freshwater fish identification Texas freshwater fish species
Texas freshwater fish species Saltwater fish in freshwater explode
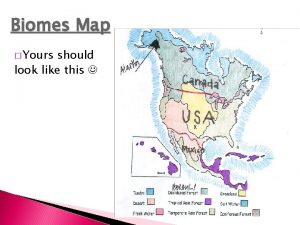
Saltwater fish in freshwater explode Map of freshwater biome
Map of freshwater biome Temperate region
Temperate region Tpc kapal adalah
Tpc kapal adalah Open water
Open water Section 1: freshwater ecosystems
Section 1: freshwater ecosystems Part part whole
Part part whole The phase of the moon you see depends on ______.
The phase of the moon you see depends on ______. Technical descriptions
Technical descriptions Minitab adalah
Minitab adalah Addition symbol
Addition symbol Parts of a bar graph
Parts of a bar graph Unit ratio definition
Unit ratio definition Aquatic biotechnology
Aquatic biotechnology Aquatic ecosystems webquest
Aquatic ecosystems webquest Epiphytes plants
Epiphytes plants Lesson 3: aquatic ecosystems
Lesson 3: aquatic ecosystems Stemmata insect
Stemmata insect The uppermost layer of an aquatic ecosystem
The uppermost layer of an aquatic ecosystem Model aquatic health code
Model aquatic health code Chapter 7 aquatic ecosystems test answers
Chapter 7 aquatic ecosystems test answers Differences between aquatic and terrestrial ecosystems
Differences between aquatic and terrestrial ecosystems Are bacteria autotrophs or heterotrophs
Are bacteria autotrophs or heterotrophs Pyramids of biomass
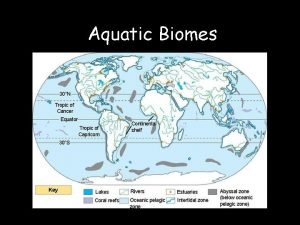
Pyramids of biomass Aquatic
Aquatic Energy pyramid in aquatic ecosystem
Energy pyramid in aquatic ecosystem Tunison laboratory of aquatic science
Tunison laboratory of aquatic science Marsilea aquatic equisetum
Marsilea aquatic equisetum Aquatic biomes in canada
Aquatic biomes in canada Examples of aquatic biomes
Examples of aquatic biomes 4-4 aquatic ecosystems
4-4 aquatic ecosystems Aquatic decomposers
Aquatic decomposers Aquatic food web examples
Aquatic food web examples Aquatic food chain
Aquatic food chain The main limiting factors in an aquatic biome are
The main limiting factors in an aquatic biome are Aquatic biomes examples
Aquatic biomes examples Terrestrial vs aquatic food production systems
Terrestrial vs aquatic food production systems Describe aquatic ecosystem
Describe aquatic ecosystem Interest grabber
Interest grabber Connecting the concepts aquatic biomes
Connecting the concepts aquatic biomes Chapter 6 biomes and aquatic ecosystems
Chapter 6 biomes and aquatic ecosystems Protista
Protista