Advances in Thermophysical Property Prediction Peiming Wang Ronald






- Slides: 38

Advances in Thermophysical Property Prediction Peiming Wang Ronald Springer Margaret Lencka Robert Young Jerzy Kosinski Andre Anderko 24 th Conference October 23 -24, 2007 THINK SIMULATION! 12/15/2021 Opening new doors with Chemistry

Scope • • OLI’s two thermodynamic models: aqueous and MSE Outline of the mixed-solvent electrolyte (MSE) thermodynamic model Application highlights Summary of MSE databanks Predictive character of the model Modeling transport properties • New model for thermal conductivity Model and databank development plans

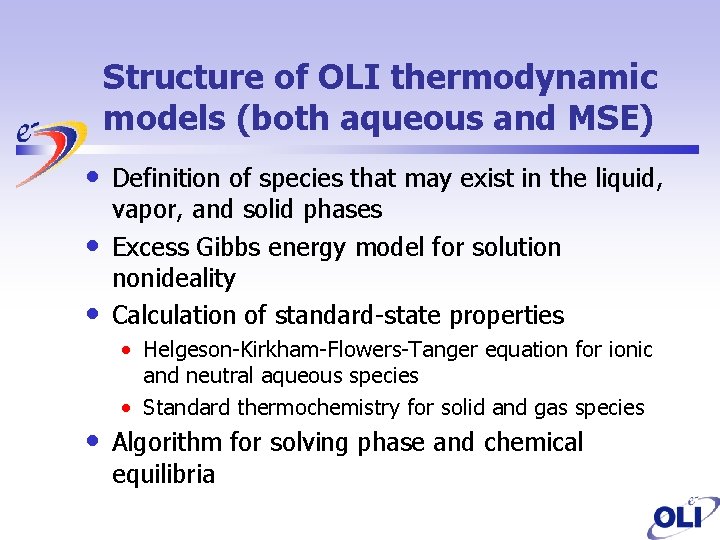
Structure of OLI thermodynamic models (both aqueous and MSE) • • Definition of species that may exist in the liquid, vapor, and solid phases Excess Gibbs energy model for solution nonideality Calculation of standard-state properties • Helgeson-Kirkham-Flowers-Tanger equation for ionic and neutral aqueous species • Standard thermochemistry for solid and gas species Algorithm for solving phase and chemical equilibria

OLI Thermodynamic Models: Aqueous and MSE • • • The difference between the models lies in • Solution nonideality model • Methodology for defining and regressing parameters Aqueous model • Solution nonideality model suitable for solutions with ionic strength below ~30 molal and nonelectrolyte mole fraction below ~0. 3 • Extensive track record and large databank MSE model • Solution nonideality model eliminates composition limitations • Development started in 2000 and model became commercial in early 2006 • Smaller, but rapidly growing databank • Includes many important systems not covered by the aqueous model

MSE Framework • Thermophysical framework to calculate • Phase equilibria and other properties in aqueous and mixed-solvent electrolyte systems n n n Electrolytes from infinite dilution to the fused-salt limit Aqueous, non-aqueous and mixed solvents Temperatures up to 0. 9 critical temperature of the system • Chemical equilibria n n Speciation of ionic solutions Reactions in solid-liquid systems

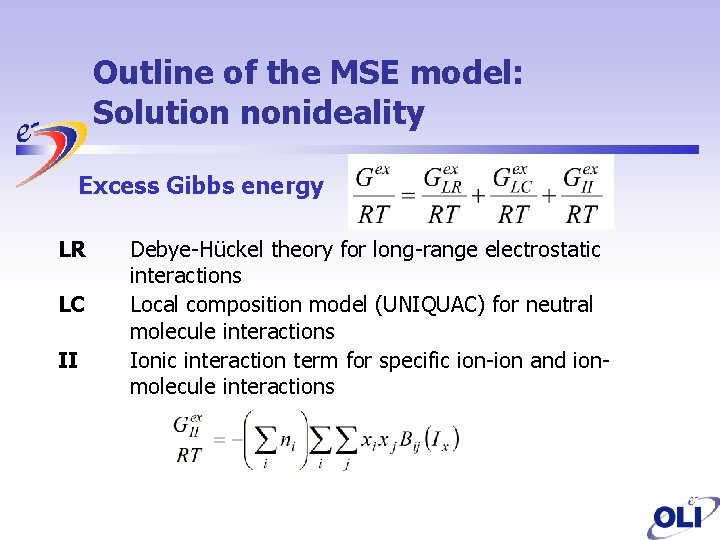
Outline of the MSE model: Solution nonideality Excess Gibbs energy LR LC II Debye-Hückel theory for long-range electrostatic interactions Local composition model (UNIQUAC) for neutral molecule interactions Ionic interaction term for specific ion-ion and ionmolecule interactions

MSE thermodynamic model: Application highlights • • • Predicting deliquescence of Na – K – Mg – Ca – Cl – NO 3 brines • Challenge: Simultaneous representation of water activity and solubility for concentrated multicomponent solutions based on parameters determined from binary and selected ternary data Phase behavior of borate systems • Challenge: Complexity of SLE patterns; multiple phases Properties of transition metal systems • Challenge: Interplay between speciation and phase behavior

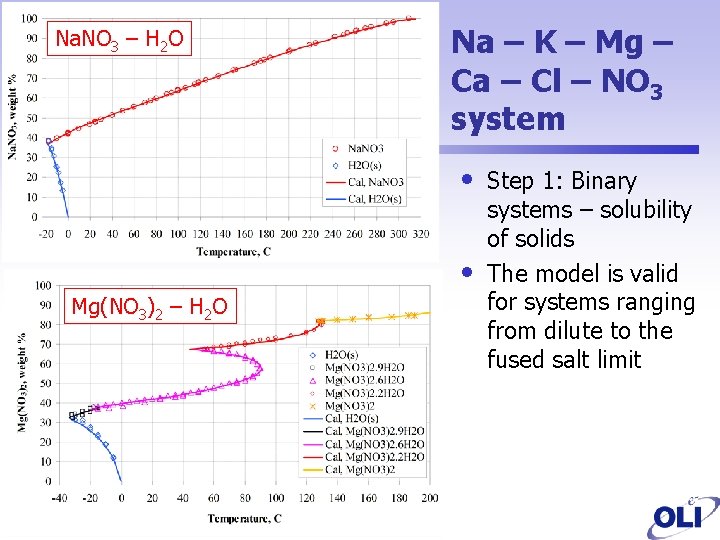
Na. NO 3 – H 2 O Na – K – Mg – Ca – Cl – NO 3 system • • Mg(NO 3)2 – H 2 O Step 1: Binary systems – solubility of solids The model is valid for systems ranging from dilute to the fused salt limit

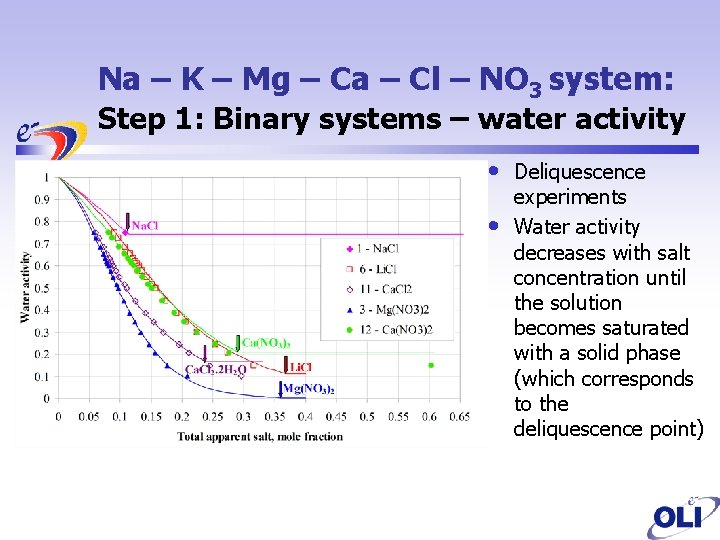
Na – K – Mg – Ca – Cl – NO 3 system: Step 1: Binary systems – water activity • • Deliquescence experiments Water activity decreases with salt concentration until the solution becomes saturated with a solid phase (which corresponds to the deliquescence point)

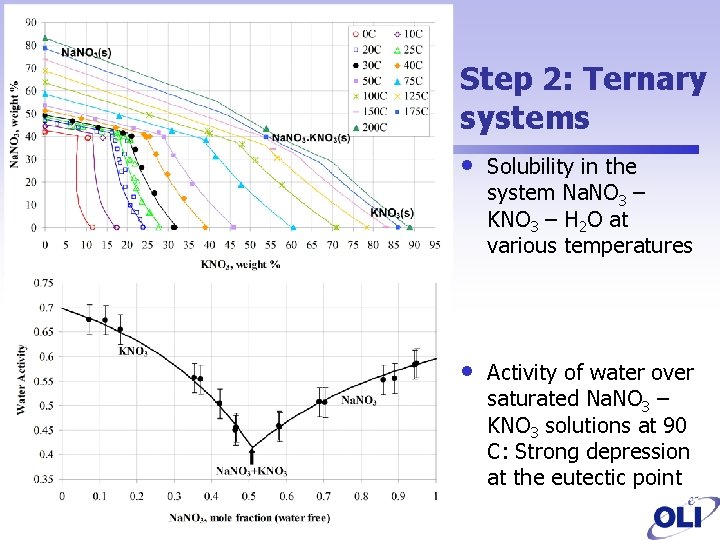
Step 2: Ternary systems • Solubility in the system Na. NO 3 – KNO 3 – H 2 O at various temperatures • Activity of water over saturated Na. NO 3 – KNO 3 solutions at 90 C: Strong depression at the eutectic point

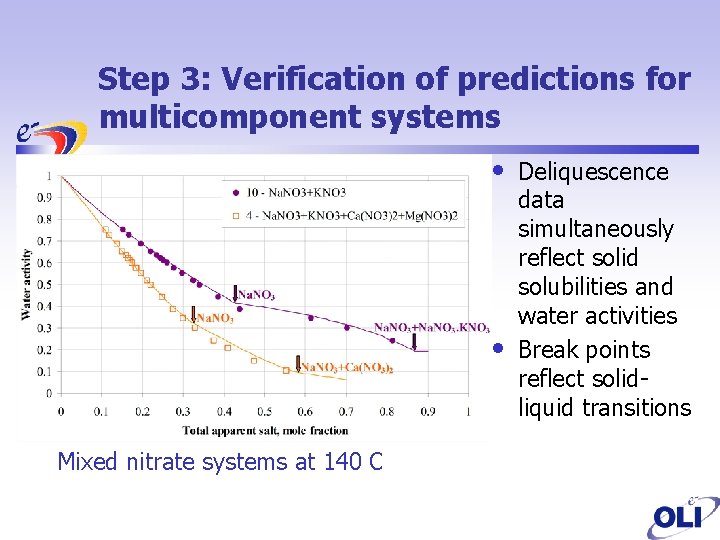
Step 3: Verification of predictions for multicomponent systems • • Mixed nitrate systems at 140 C Deliquescence data simultaneously reflect solid solubilities and water activities Break points reflect solidliquid transitions

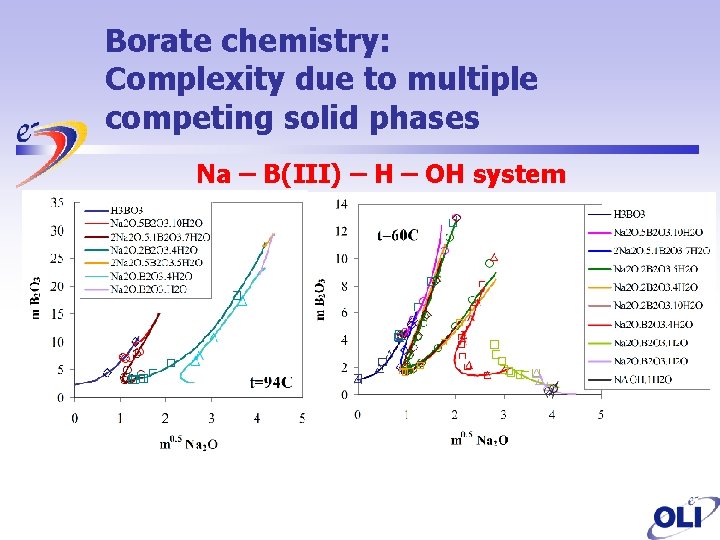
Borate chemistry: Complexity due to multiple competing solid phases Na – B(III) – H – OH system

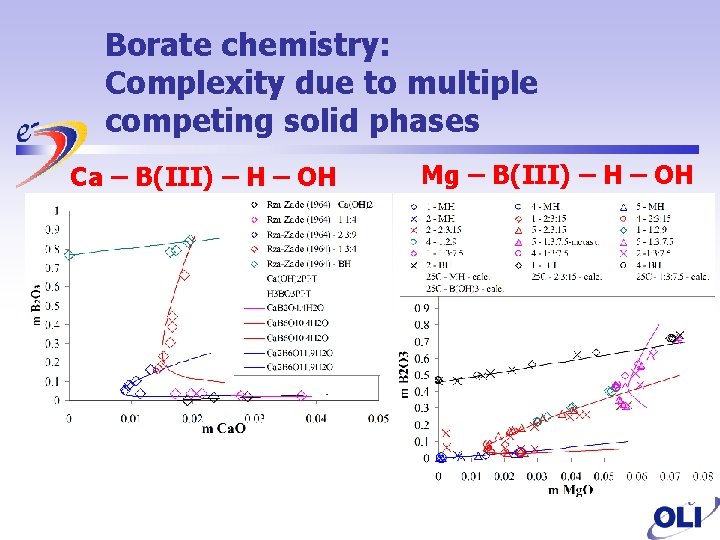
Borate chemistry: Complexity due to multiple competing solid phases Ca – B(III) – H – OH Mg – B(III) – H – OH

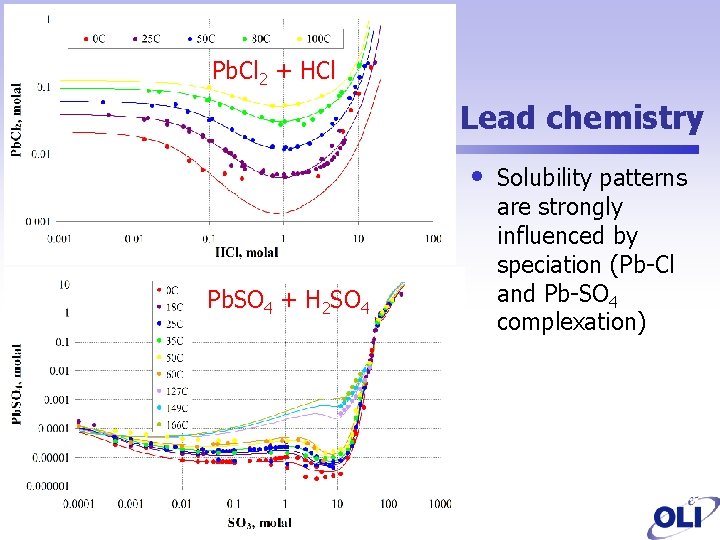
Pb. Cl 2 + HCl Lead chemistry • Pb. SO 4 + H 2 SO 4 Solubility patterns are strongly influenced by speciation (Pb-Cl and Pb-SO 4 complexation)

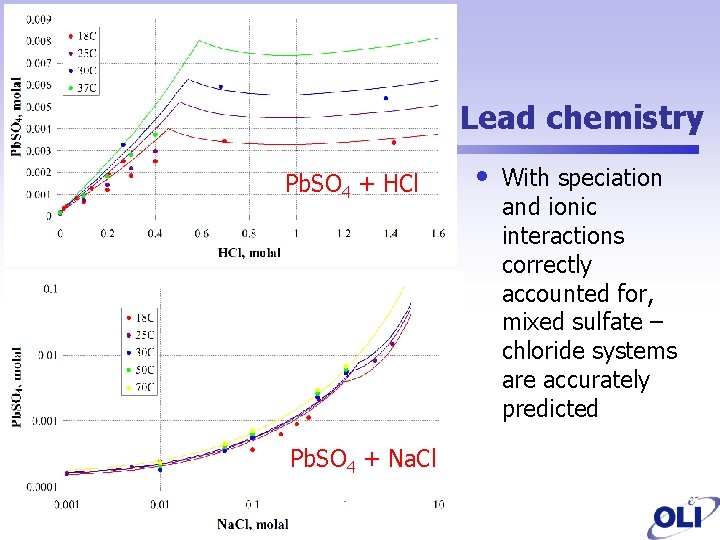
Lead chemistry Pb. SO 4 + HCl Pb. SO 4 + Na. Cl • With speciation and ionic interactions correctly accounted for, mixed sulfate – chloride systems are accurately predicted

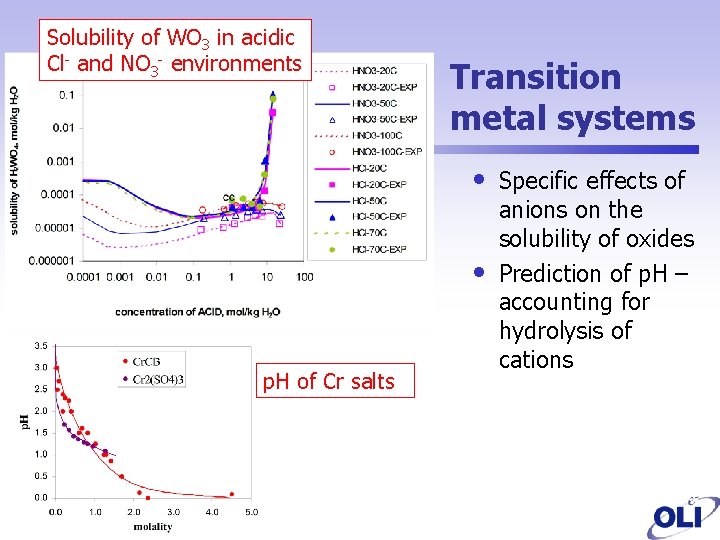
Solubility of WO 3 in acidic Cl- and NO 3 - environments Transition metal systems • • p. H of Cr salts Specific effects of anions on the solubility of oxides Prediction of p. H – accounting for hydrolysis of cations

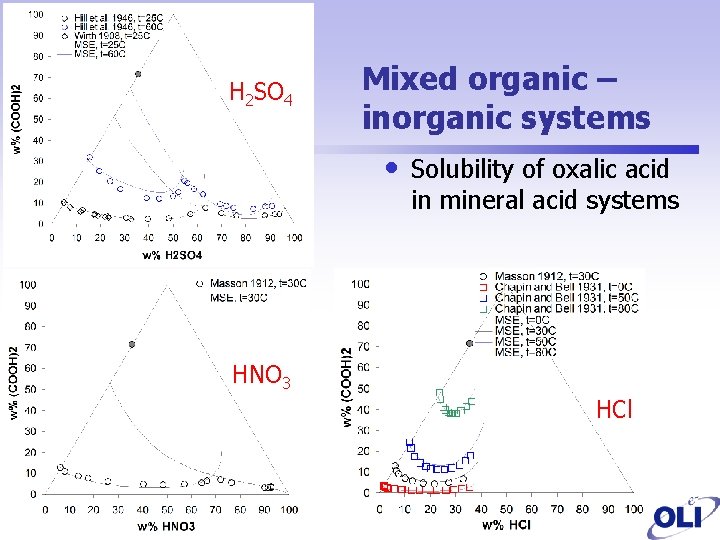
H 2 SO 4 Mixed organic – inorganic systems • Solubility of oxalic acid in mineral acid systems HNO 3 HCl

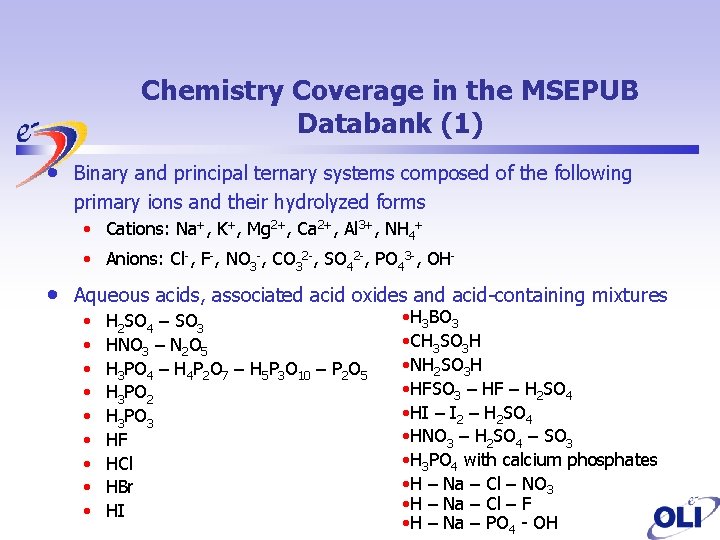
Chemistry Coverage in the MSEPUB Databank (1) • Binary and principal ternary systems composed of the following primary ions and their hydrolyzed forms • Cations: Na+, K+, Mg 2+, Ca 2+, Al 3+, NH 4+ • Anions: Cl-, F-, NO 3 -, CO 32 -, SO 42 -, PO 43 -, OH- • Aqueous acids, associated acid oxides and acid-containing mixtures • • • H 2 SO 4 – SO 3 HNO 3 – N 2 O 5 H 3 PO 4 – H 4 P 2 O 7 – H 5 P 3 O 10 – P 2 O 5 H 3 PO 2 H 3 PO 3 HF HCl HBr HI • H 3 BO 3 • CH 3 SO 3 H • NH 2 SO 3 H • HFSO 3 – HF – H 2 SO 4 • HI – I 2 – H 2 SO 4 • HNO 3 – H 2 SO 4 – SO 3 • H 3 PO 4 with calcium phosphates • H – Na – Cl – NO 3 • H – Na – Cl – F • H – Na – PO 4 - OH

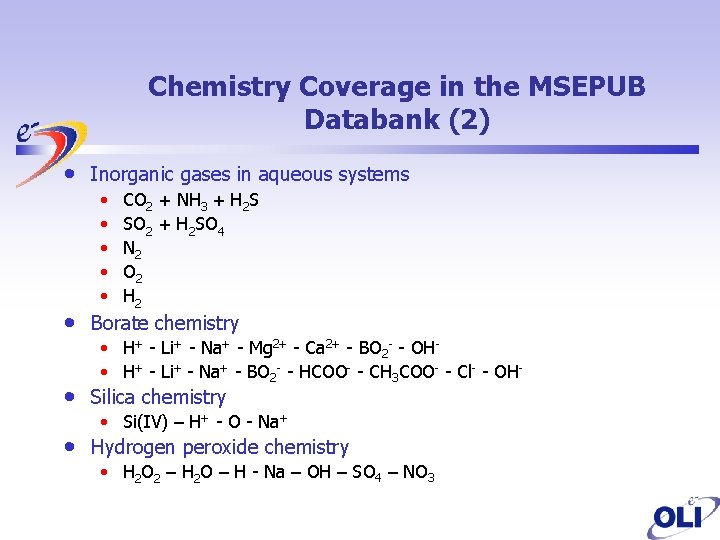
Chemistry Coverage in the MSEPUB Databank (2) • • Inorganic gases in aqueous systems • • • CO 2 + NH 3 + H 2 S SO 2 + H 2 SO 4 N 2 O 2 H 2 Borate chemistry • H+ - Li+ - Na+ - Mg 2+ - Ca 2+ - BO 2 - - OH • H+ - Li+ - Na+ - BO 2 - - HCOO- - CH 3 COO- - Cl- - OH- Silica chemistry • Si(IV) – H+ - O - Na+ Hydrogen peroxide chemistry • H 2 O 2 – H 2 O – H - Na – OH – SO 4 – NO 3

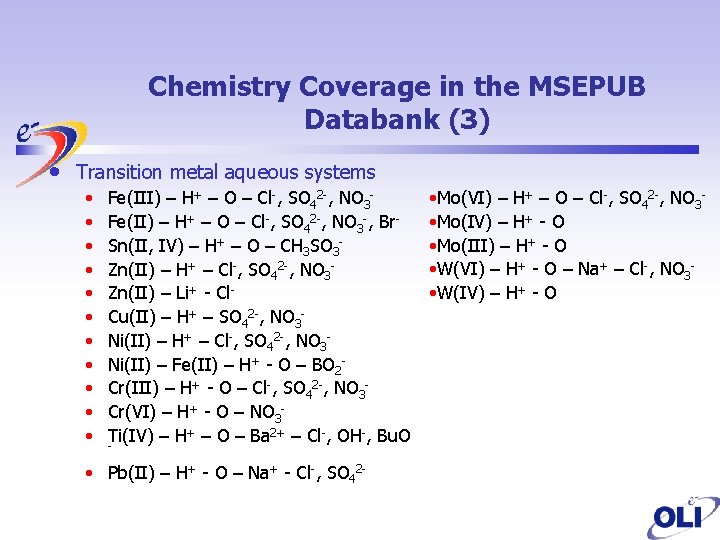
Chemistry Coverage in the MSEPUB Databank (3) • Transition metal aqueous systems • • • Fe(III) – H+ – O – Cl-, SO 42 -, NO 3 Fe(II) – H+ – O – Cl-, SO 42 -, NO 3 -, Br. Sn(II, IV) – H+ – O – CH 3 SO 3 Zn(II) – H+ – Cl-, SO 42 -, NO 3 Zn(II) – Li+ - Cl. Cu(II) – H+ – SO 42 -, NO 3 Ni(II) – H+ – Cl-, SO 42 -, NO 3 Ni(II) – Fe(II) – H+ - O – BO 2 Cr(III) – H+ - O – Cl-, SO 42 -, NO 3 Cr(VI) – H+ - O – NO 3 Ti(IV) – H+ – O – Ba 2+ – Cl-, OH-, Bu. O - • Pb(II) – H+ - O – Na+ - Cl-, SO 42 - • Mo(VI) – H+ – O – Cl-, SO 42 -, NO 3 • Mo(IV) – H+ - O • Mo(III) – H+ - O • W(VI) – H+ - O – Na+ – Cl-, NO 3 • W(IV) – H+ - O

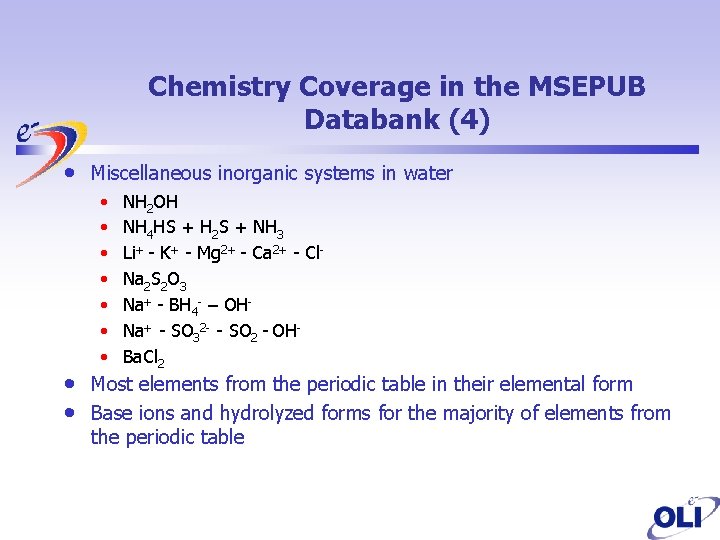
Chemistry Coverage in the MSEPUB Databank (4) • • • Miscellaneous inorganic systems in water • • NH 2 OH NH 4 HS + H 2 S + NH 3 Li+ - K+ - Mg 2+ - Ca 2+ - Cl. Na 2 S 2 O 3 Na+ - BH 4 - – OHNa+ - SO 32 - - SO 2 - OHBa. Cl 2 Most elements from the periodic table in their elemental form Base ions and hydrolyzed forms for the majority of elements from the periodic table

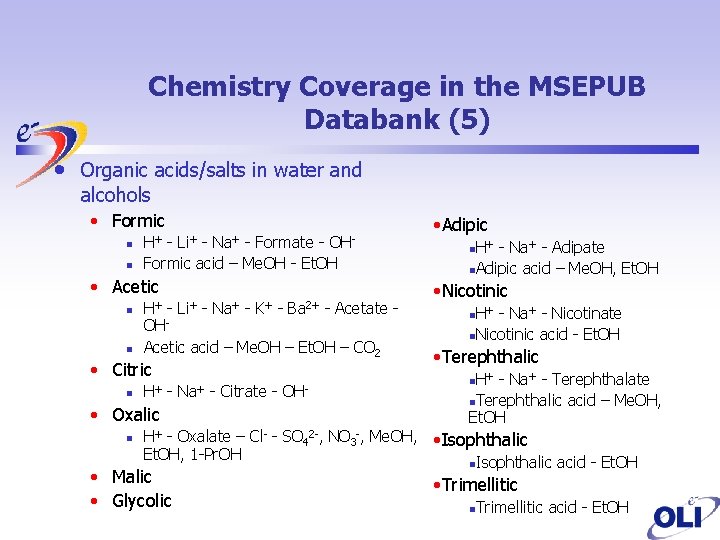
Chemistry Coverage in the MSEPUB Databank (5) • Organic acids/salts in water and alcohols • Formic n n H+ Li+ Na+ OH- - Formate Formic acid – Me. OH - Et. OH • Acetic n H+ - n Acetic acid – Me. OH – Et. OH – CO 2 OH- - - K+ - Ba 2+ • Citric n H+ - Na+ - Citrate - OH- • Oxalic n - Acetate - • Adipic H+ - Na+ - Adipate n. Adipic acid – Me. OH, Et. OH n • Nicotinic H+ - Na+ - Nicotinate n. Nicotinic acid - Et. OH n • Terephthalic H+ - Na+ - Terephthalate n. Terephthalic acid – Me. OH, Et. OH n H+ - Oxalate – Cl- - SO 42 -, NO 3 -, Me. OH, • Isophthalic Et. OH, 1 -Pr. OH n. Isophthalic acid - Et. OH • Malic • Glycolic • Trimellitic n Trimellitic acid - Et. OH

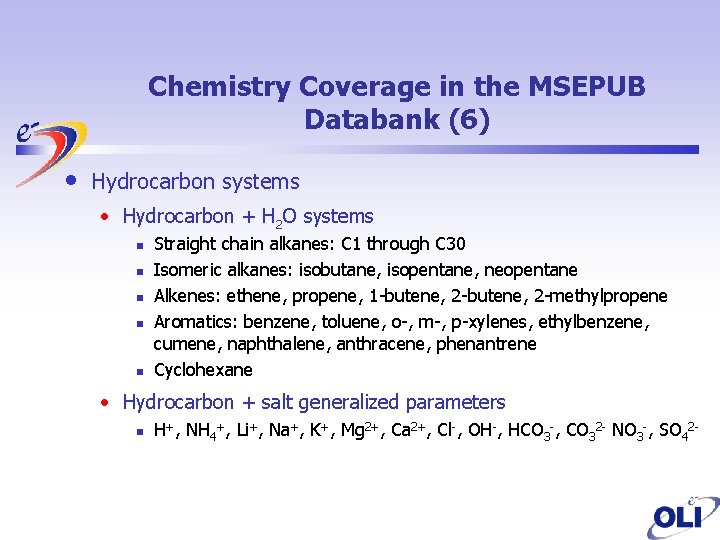
Chemistry Coverage in the MSEPUB Databank (6) • Hydrocarbon systems • Hydrocarbon + H 2 O systems n n n Straight chain alkanes: C 1 through C 30 Isomeric alkanes: isobutane, isopentane, neopentane Alkenes: ethene, propene, 1 -butene, 2 -methylpropene Aromatics: benzene, toluene, o-, m-, p-xylenes, ethylbenzene, cumene, naphthalene, anthracene, phenantrene Cyclohexane • Hydrocarbon + salt generalized parameters n H+, NH 4+, Li+, Na+, K+, Mg 2+, Ca 2+, Cl-, OH-, HCO 3 -, CO 32 - NO 3 -, SO 42 -

Chemistry Coverage in the MSEPUB Databank (7) • Organic solvents and their mixtures with water • Alcohols n Methanol, 1 -propanol, 2 -propanol, 1 -butanol, cyclohexanol • Glycols n Mono, di- and triethylene glycols, propylene glycol, polyethylene glycols • Phenols n Phenol, catechol • Ketones n Acetone, methylisobutyl ketone • Aldehydes n Butylaldehyde • Carbonates n Diethylcarbonate, propylene carbonate

Chemistry Coverage in the MSEPUB Databank (8) • Organic solvents and their mixtures with water • Amines n Tri-N-octylamine, triethylamine, methyldiethanolamine • Nitriles n Acetonitrile • Amides n Dimethylacetamide, dimethylformamide • Halogen derivatives n Chloroform, carbon tetrachloride • Aminoacids n Methionine • Heterocyclic components n N-methylpyrrolidone, 2, 6 -dimethylmorpholine

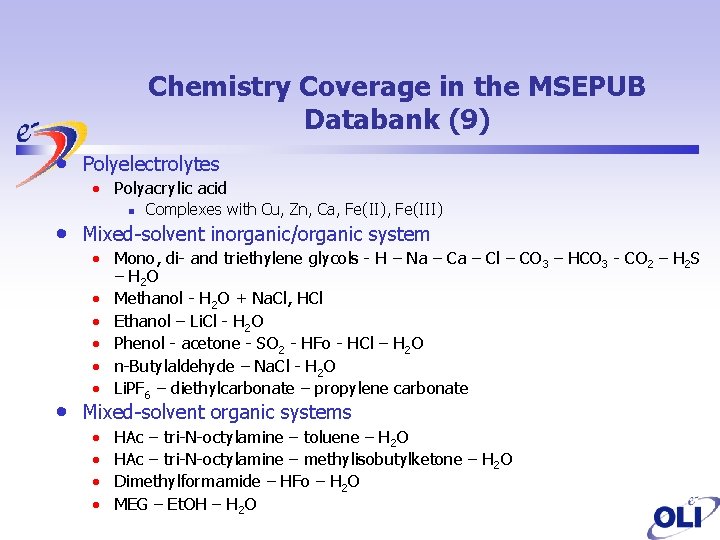
Chemistry Coverage in the MSEPUB Databank (9) • Polyelectrolytes • Polyacrylic acid • • n Complexes with Cu, Zn, Ca, Fe(II), Fe(III) Mixed-solvent inorganic/organic system • Mono, di- and triethylene glycols - H – Na – Cl – CO 3 – HCO 3 - CO 2 – H 2 S – H 2 O • Methanol - H 2 O + Na. Cl, HCl • Ethanol – Li. Cl - H 2 O • Phenol - acetone - SO 2 - HFo - HCl – H 2 O • n-Butylaldehyde – Na. Cl - H 2 O • Li. PF 6 – diethylcarbonate – propylene carbonate Mixed-solvent organic systems • • HAc – tri-N-octylamine – toluene – H 2 O HAc – tri-N-octylamine – methylisobutylketone – H 2 O Dimethylformamide – HFo – H 2 O MEG – Et. OH – H 2 O

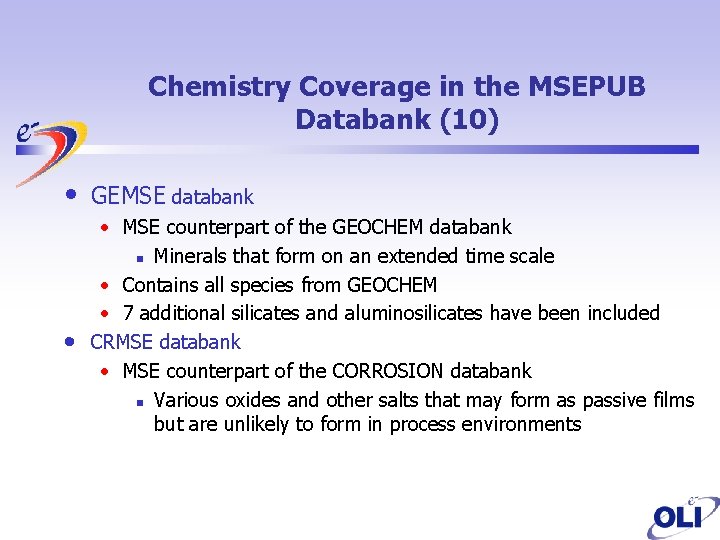
Chemistry Coverage in the MSEPUB Databank (10) • • GEMSE databank • MSE counterpart of the GEOCHEM databank n Minerals that form on an extended time scale • Contains all species from GEOCHEM • 7 additional silicates and aluminosilicates have been included CRMSE databank • MSE counterpart of the CORROSION databank n Various oxides and other salts that may form as passive films but are unlikely to form in process environments

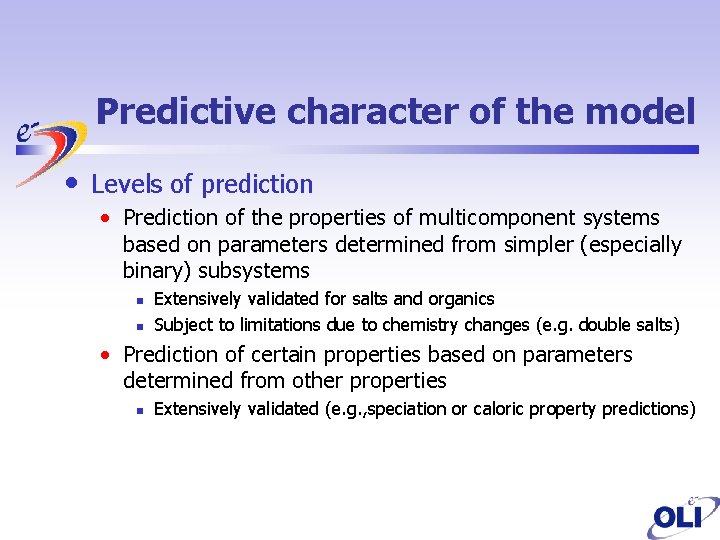
Predictive character of the model • Levels of prediction • Prediction of the properties of multicomponent systems based on parameters determined from simpler (especially binary) subsystems n n Extensively validated for salts and organics Subject to limitations due to chemistry changes (e. g. double salts) • Prediction of certain properties based on parameters determined from other properties n Extensively validated (e. g. , speciation or caloric property predictions)

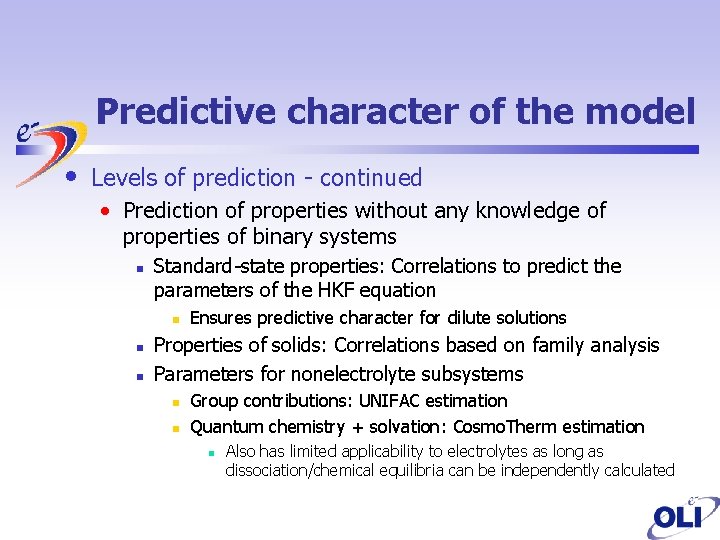
Predictive character of the model • Levels of prediction - continued • Prediction of properties without any knowledge of properties of binary systems n Standard-state properties: Correlations to predict the parameters of the HKF equation n Ensures predictive character for dilute solutions Properties of solids: Correlations based on family analysis Parameters for nonelectrolyte subsystems n n Group contributions: UNIFAC estimation Quantum chemistry + solvation: Cosmo. Therm estimation n Also has limited applicability to electrolytes as long as dissociation/chemical equilibria can be independently calculated

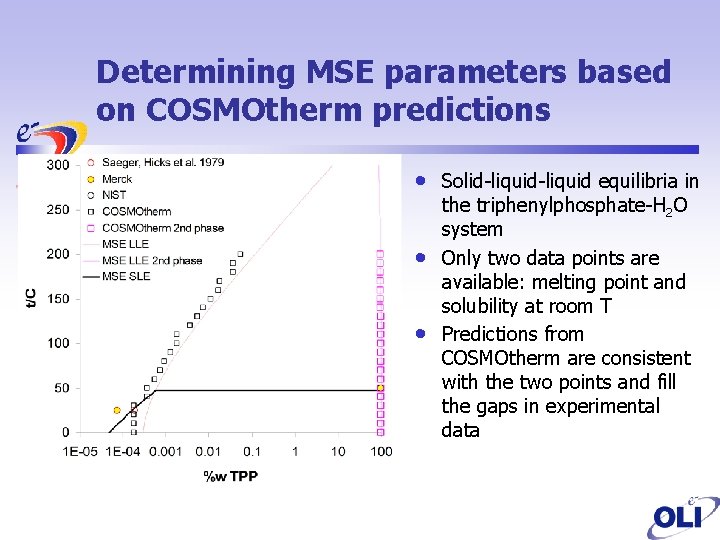
Determining MSE parameters based on COSMOtherm predictions • • • Solid-liquid equilibria in the triphenylphosphate-H 2 O system Only two data points are available: melting point and solubility at room T Predictions from COSMOtherm are consistent with the two points and fill the gaps in experimental data

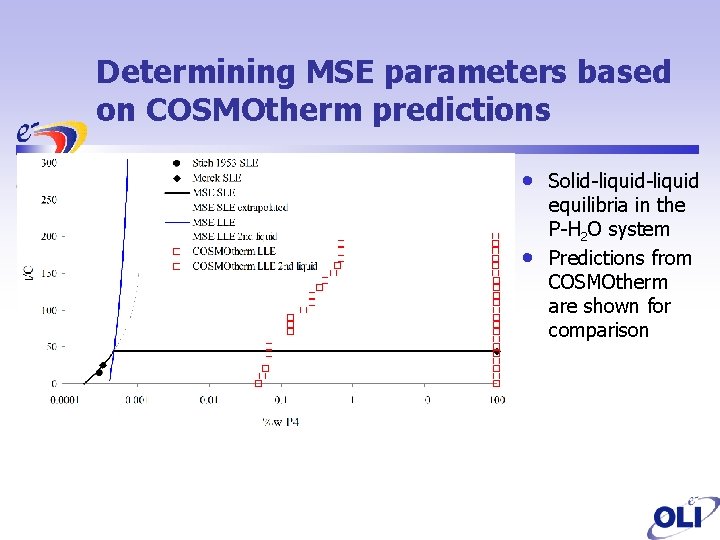
Determining MSE parameters based on COSMOtherm predictions • • Solid-liquid equilibria in the P-H 2 O system Predictions from COSMOtherm are shown for comparison

Transport properties in the OLI software • • • Available transport properties: • Diffusivity • Viscosity • Electrical conductivity These models were developed first in conjunction with the aqueous model and then extended to mixedsolvent systems A new model for calculating thermal conductivity has been recently developed

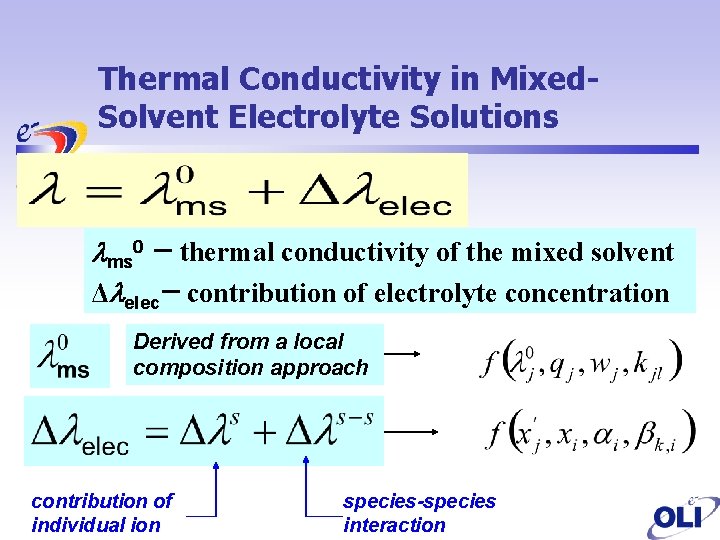
Thermal Conductivity in Mixed. Solvent Electrolyte Solutions lms 0 thermal conductivity of the mixed solvent Δlelec contribution of electrolyte concentration Derived from a local composition approach contribution of individual ion species-species interaction

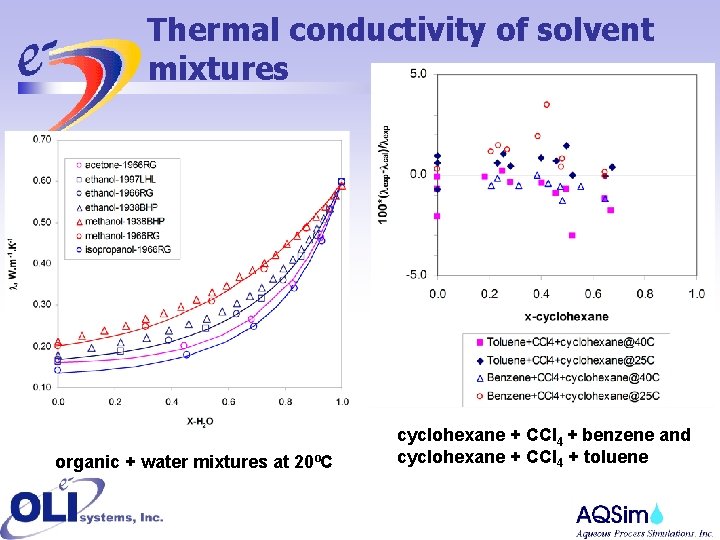
Thermal conductivity of solvent mixtures organic + water mixtures at 20ºC cyclohexane + CCl 4 + benzene and cyclohexane + CCl 4 + toluene

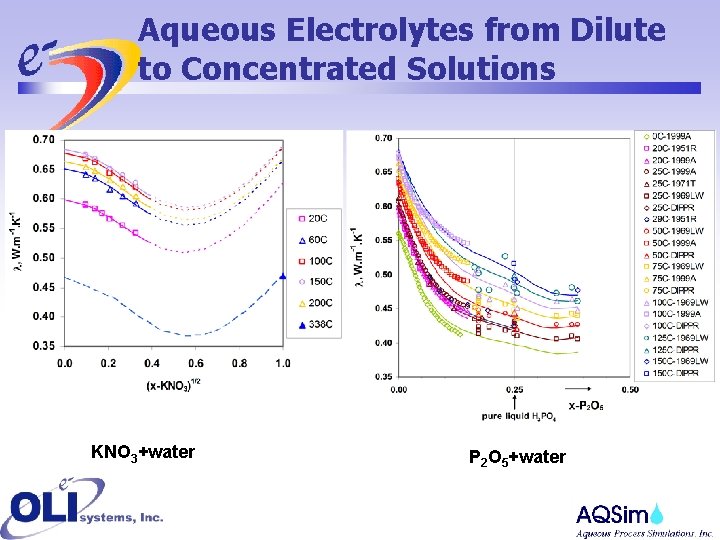
Aqueous Electrolytes from Dilute to Concentrated Solutions KNO 3+water P 2 O 5+water

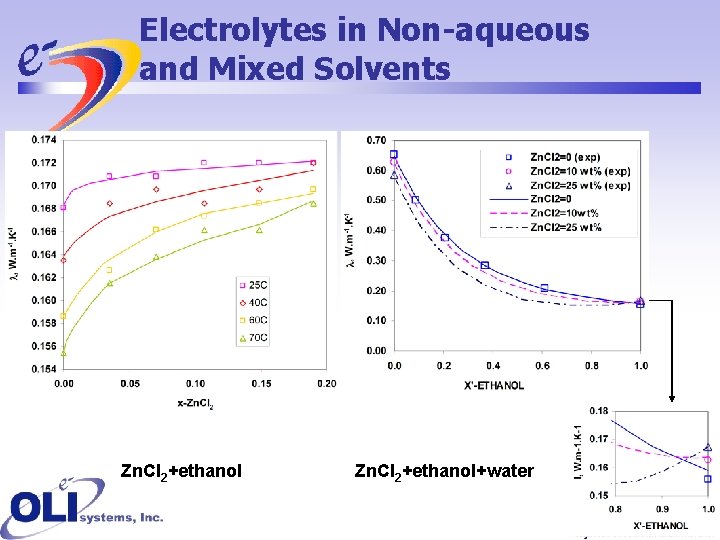
Electrolytes in Non-aqueous and Mixed Solvents Zn. Cl 2+ethanol+water

Further Development of MSE • • Thermophysical property models • Implementation of thermal conductivity in OLI software • Development of a surface tension model Major parameter development projects • Refinery overhead consortium (in collaboration with Sw. RI) n Development of parameters for amines and amine hydrochlorides • Hanford tank chemistry in MSE • Modeling hydrometallurgical systems (University of Toronto) • Transition metal chemistry including complexation • Natural water chemistry (including common scales) with methanol and glycols • Urea chemistry • Other projects as defined by clients

Summary • OLI’s two thermophysical property packages • Mixed-solvent electrolyte model n n Thermophysical engine for the future General, accurate framework for reproducing the properties of electrolyte and nonelectrolyte systems without concentration limits over wide ranges of conditions Parameter databanks are being rapidly expanded New thermophysical properties (thermal conductivity, surface tension) are being added • Aqueous model n n Widely used and reliable Continues to be maintained and parameters continue to be added as requested by clients
 Thermal conductivity of dental materials
Thermal conductivity of dental materials Short term loans and advances
Short term loans and advances Lurbinectedin posologie
Lurbinectedin posologie Child development chapter 9
Child development chapter 9 Opto-electronic advances
Opto-electronic advances Axis powers
Axis powers Advances in memory technology
Advances in memory technology Advances in real-time rendering in games
Advances in real-time rendering in games Recent advances in ceramics
Recent advances in ceramics Tally solutions pvt ltd
Tally solutions pvt ltd Coherent scattering
Coherent scattering Asset classification norms
Asset classification norms Advances in technology during wwii
Advances in technology during wwii Sapratibandha daya and apratibandha daya
Sapratibandha daya and apratibandha daya Commutative property vs associative property
Commutative property vs associative property Chemical properties of citric acid
Chemical properties of citric acid Ronald barzola
Ronald barzola Ronald rahmig
Ronald rahmig Abnormal psychology chapter 5
Abnormal psychology chapter 5 Maxwell relations
Maxwell relations Ronald van marlen
Ronald van marlen Ronald reagan reaganomics
Ronald reagan reaganomics Ronald lee in advancing
Ronald lee in advancing Ronald mitsuyasu
Ronald mitsuyasu Dr eran (ronald) lev
Dr eran (ronald) lev Snaar theorie
Snaar theorie Dochters ids postma
Dochters ids postma Ronald p puttense moordzaak
Ronald p puttense moordzaak Dr ronald melles
Dr ronald melles Clasificacion johner y wruhs
Clasificacion johner y wruhs Internalizacio
Internalizacio Abnormal psychology comer 9th edition
Abnormal psychology comer 9th edition Ronald klasko
Ronald klasko Ronald h brown ship
Ronald h brown ship Ron schouten
Ron schouten Ronald mathieu
Ronald mathieu Ronald wyatt md
Ronald wyatt md Jimmy carter ducksters
Jimmy carter ducksters Ronald morgan goes to bat
Ronald morgan goes to bat