ACIDS ACIDS BASES BASES SALTS Acids verses Bases

ACIDS, ACIDS BASES, BASES & SALTS

Acids verses Bases Properties of ACIDS: Properties of BASES: 1. 2. 3. 1. bitter taste 2. classified as electrolyte 3. react with acids (neutralization rxn) 4. slippery or soapy feeling 1. bases turn litmus paper blue 4. 5. sour taste classified as electrolyte react with bases (neutralization reaction) react with most metals to produce H 2(g) (SR rxn) acids turn litmus paper red
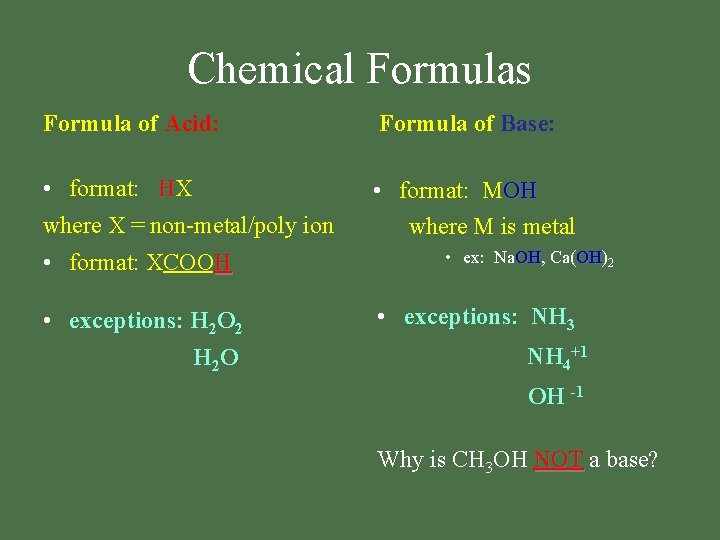
Chemical Formulas Formula of Acid: Formula of Base: • format: HX where X = non-metal/poly ion • format: MOH where M is metal • format: XCOOH • exceptions: H 2 O 2 H 2 O • ex: Na. OH, OH Ca(OH) OH 2 • exceptions: NH 3 NH 4+1 OH -1 Why is CH 3 OH NOT a base?

Which metals react with acids? • see Table J: all metals above H 2 react with acids – undergo SR rxns (metal replaces H in acid) • Cu, Ag, and Au do NOT react with acids

Electrolyte • substance that dissolves in H 2 O to produce aqueous soln that conducts electric current • 3 substances fit this description: acids, bases and salts

Electrolytes All 3 subs dissociate (ionize) in H 2 O(l) ACID: HCl(s) H+1(aq) + Cl-1(aq) H 2 O(l) BASE: Na. OH(s) Na+1(aq) + OH-1(aq) H 2 O(l) SALT: Na. Cl(s) Na+1(aq) + Cl-1(aq)

Identify the Electrolytes Yes - salt NO • C 2 H 5 OH • Na. Cl • CCl 4 NO • HNO 3 Yes - acid • H 2 SO 4 • C 5 H 12 NO Yes - acid • Na. OH Yes – base & salt • K 3 PO 4 Yes - salt • C 6 H 12 O 6 NO • CH 3 OCH 3 NO • Ca. I 2 Yes - salt • Li. OH Yes – salt & base • HF Yes - acid • HI Yes - acid • Mg(OH)2 Yes – base & salt • (NH 4)2 SO 4 Yes - base • C 3 H 7 OH NO • C 12 H 22 O 11 NO

Acid-Base Theories: • Arrhenius Theory • Bronstead-Lowry Theory – acids/bases ionize in – aka: alternative theory water – based on type ions – based on acid/base produced when being proton acid/base mixed donor/receiver with water – limitations – NO limitations
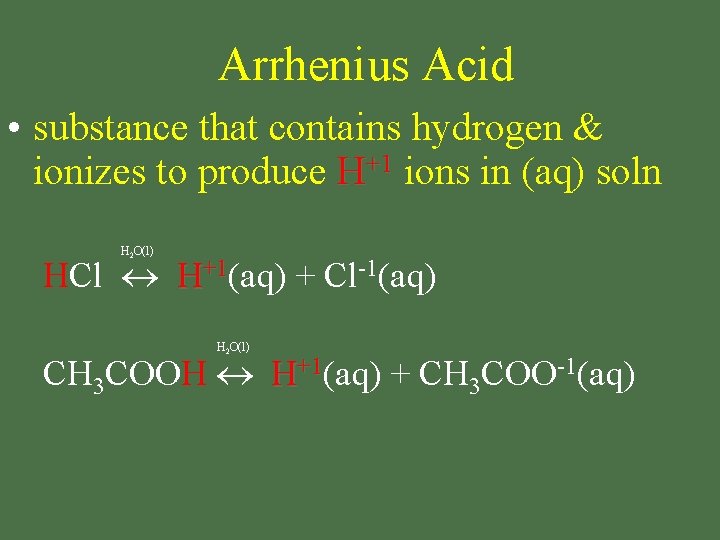
Arrhenius Acid • substance that contains hydrogen & ionizes to produce H+1 ions in (aq) soln H 2 O(l) HCl H+1(aq) + Cl-1(aq) H 2 O(l) CH 3 COOH H+1(aq) + CH 3 COO-1(aq)
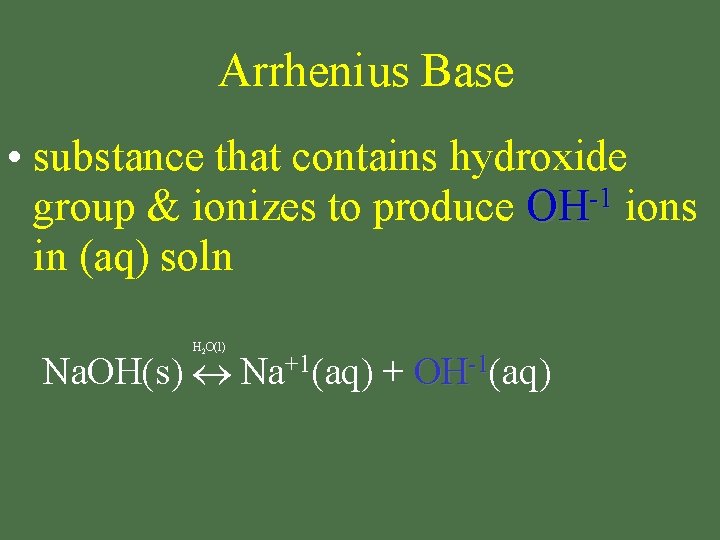
Arrhenius Base • substance that contains hydroxide -1 group & ionizes to produce OH ions in (aq) soln H 2 O(l) Na. OH(s) Na+1(aq) + OH-1(aq)

Arrhenius Salt • electrolyte where not only (+) ion and OH-1 not only (-) ion formed in aqueous solution ex: +1 H H 2 O(l) Ca. Br 2(s) Ca+2(aq) + 2 Br-1(aq) H 2 O(l) KNO 3(s) K+1(aq) + NO 3 -1(aq)
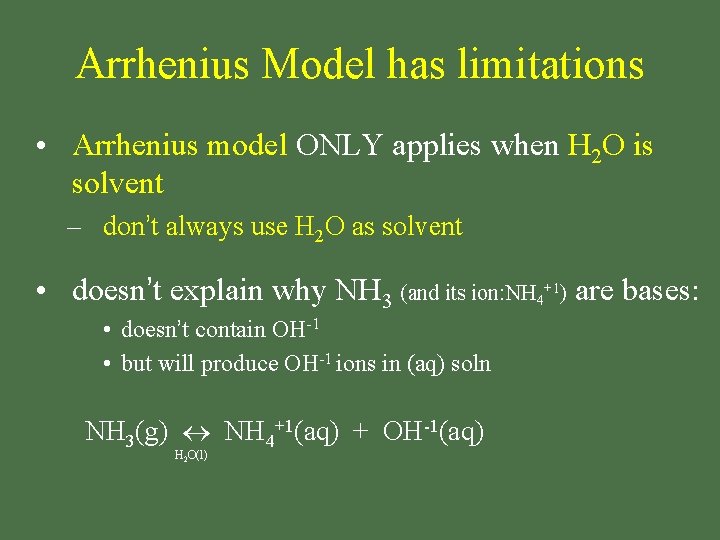
Arrhenius Model has limitations • Arrhenius model ONLY applies when H 2 O is solvent – don’t always use H 2 O as solvent • doesn’t explain why NH 3 (and its ion: NH 4+1) are bases: • doesn’t contain OH-1 • but will produce OH-1 ions in (aq) soln NH 3(g) NH 4+1(aq) + OH-1(aq) H 2 O(l)
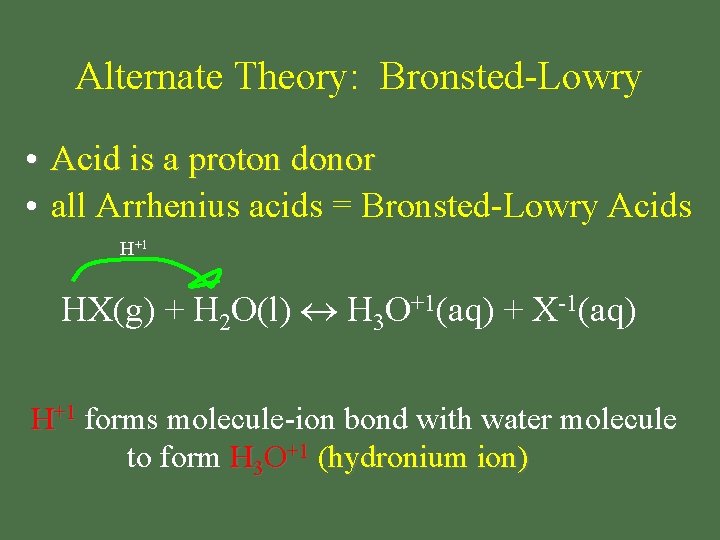
Alternate Theory: Bronsted-Lowry • Acid is a proton donor • all Arrhenius acids = Bronsted-Lowry Acids H+1 HX(g) + H 2 O(l) H 3 O+1(aq) + X-1(aq) H+1 forms molecule-ion bond with water molecule to form H 3 O+1 (hydronium ion)
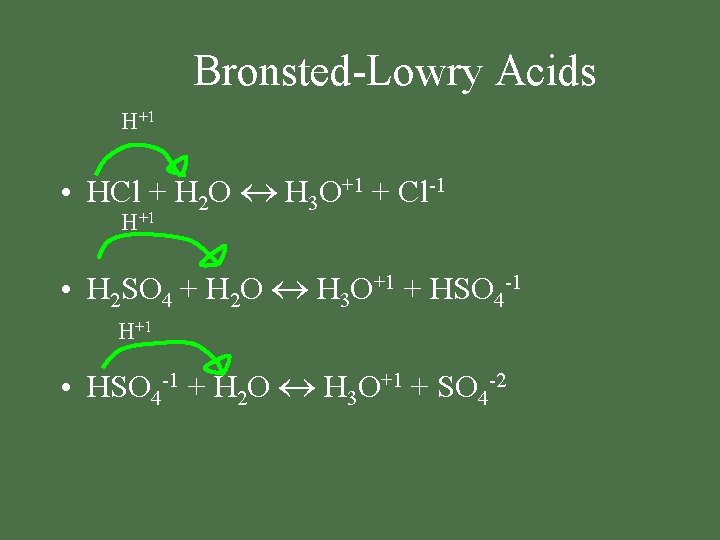
Bronsted-Lowry Acids H+1 • HCl + H 2 O H 3 O+1 + Cl-1 H+1 • H 2 SO 4 + H 2 O H 3 O+1 + HSO 4 -1 H+1 • HSO 4 -1 + H 2 O H 3 O+1 + SO 4 -2
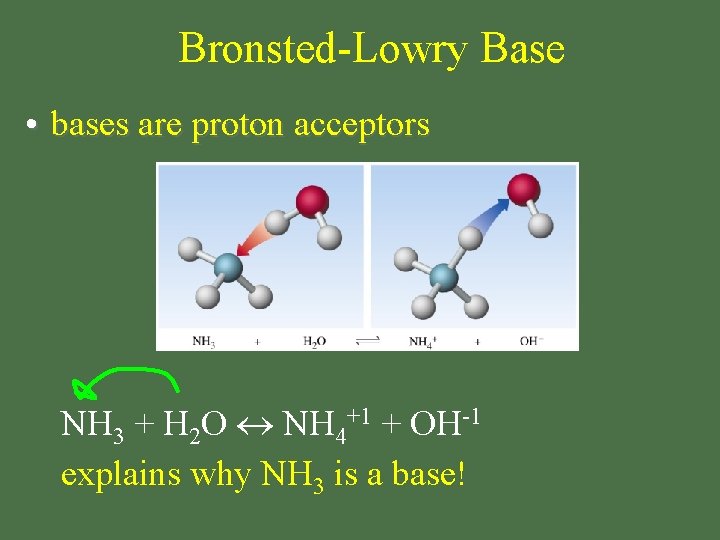
Bronsted-Lowry Base • bases are proton acceptors NH 3 + H 2 O NH 4+1 + OH-1 explains why NH 3 is a base!

Amphoteric • substance that acts as BOTH an acid & a base
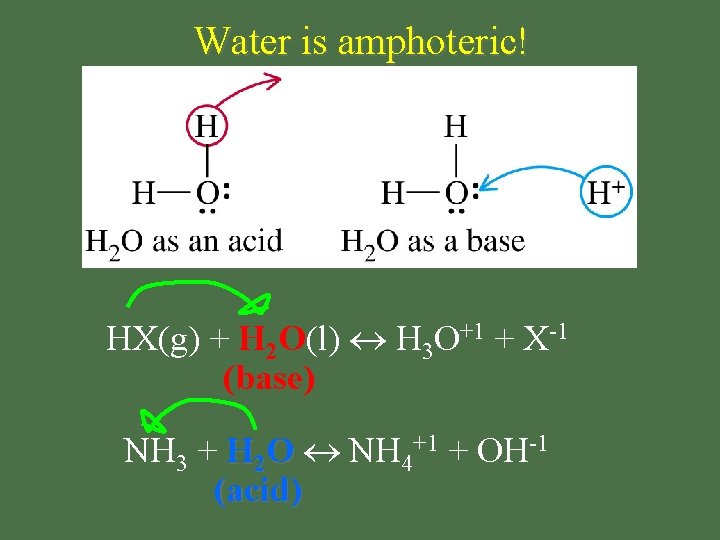
Water is amphoteric! HX(g) + H 2 O(l) H 3 O+1 + X-1 (base) NH 3 + H 2 O NH 4+1 + OH-1 (acid)
- Slides: 17