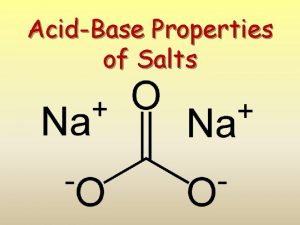
ACIDS ACIDS BASES BASES SALTS Properties of Acids

ACIDS, ACIDS BASES, BASES & SALTS

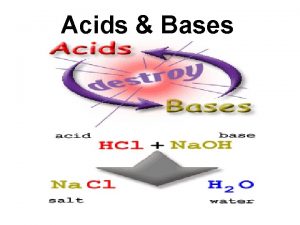
Properties of Acids 1. sour taste 2. electrolytes: - aqueous solns conduct electric current 3. react with bases to form water and salt (neutralization reaction) 4. react with most metals to produce H 2(g) 5. acids turn litmus paper red
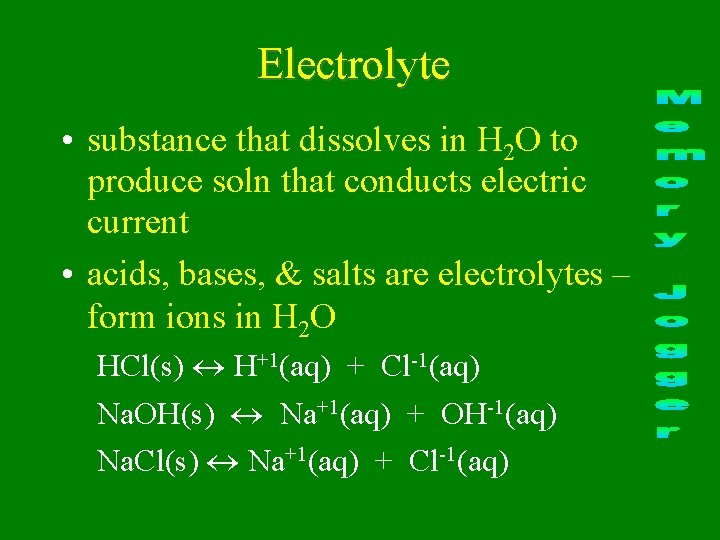
Electrolyte • substance that dissolves in H 2 O to produce soln that conducts electric current • acids, bases, & salts are electrolytes – form ions in H 2 O HCl(s) H+1(aq) + Cl-1(aq) Na. OH(s) Na+1(aq) + OH-1(aq) Na. Cl(s) Na+1(aq) + Cl-1(aq)

Which metals react with acids? • See Table J • All metals above H 2 react with acids • Cu, Ag, and Au do not react with acids
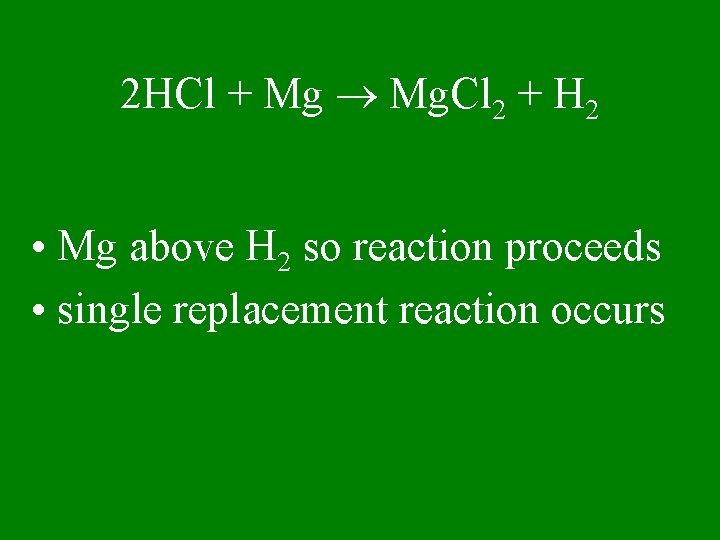
2 HCl + Mg Mg. Cl 2 + H 2 • Mg above H 2 so reaction proceeds • single replacement reaction occurs

Properties of Bases 1. 2. 3. 4. bitter taste slippery or soapy feeling Electrolytes react with acids to produce water and salt 5. bases turn litmus paper blue

Formula of Acid • Format: HX where X = nonmetal (F, Cl, Br, I) or X = negative polyatomic ion • some acids have 2 or 3 H’s – Ex: HF, H 2 S, H 3 PO 4

Formula of Base • Format: MOH where M is metal • Ex: Na. OH, OH Ca(OH) OH 2 – exception: NH 3 and NH 4+1 • CH 3 OH is NOT a base. WHY?
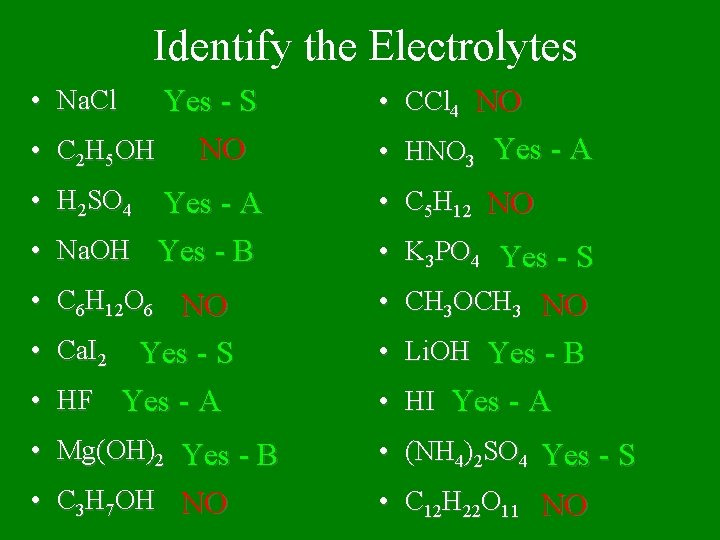
Identify the Electrolytes Yes - S NO • C 2 H 5 OH • CCl 4 NO • H 2 SO 4 • C 5 H 12 NO • C 6 H 12 O 6 NO • K 3 PO 4 Yes - S • CH 3 OCH 3 NO • Ca. I 2 Yes - S • HF Yes - A • Li. OH Yes - B • Mg(OH)2 Yes - B • (NH 4)2 SO 4 Yes - S • C 3 H 7 OH NO • C 12 H 22 O 11 NO • Na. Cl Yes - A • Na. OH Yes - B • HNO 3 Yes - A • HI Yes - A

Acid, Base, or Neutral? • all H 2 O contains some H+1 and some OH-1 ions – pure H 2 O: concentrations very low • neutral solution: [H+1] = [OH-1] • acidic solution: H+1 > OH-1 • basic solution: OH-1 > H+1

Water & self-ionization • H 2 O(l) + H 2 O(l) H 3 O+1(aq) + OH-1(aq) H 3 O+1 = hydronium ion OH-1 = hydroxide ion • H 2 O(l) H+1(aq) + OH-1(aq) H+1 and H 3 O+1 used interchangeably H+1 called proton or hydrogen ion
Self-ionization of water
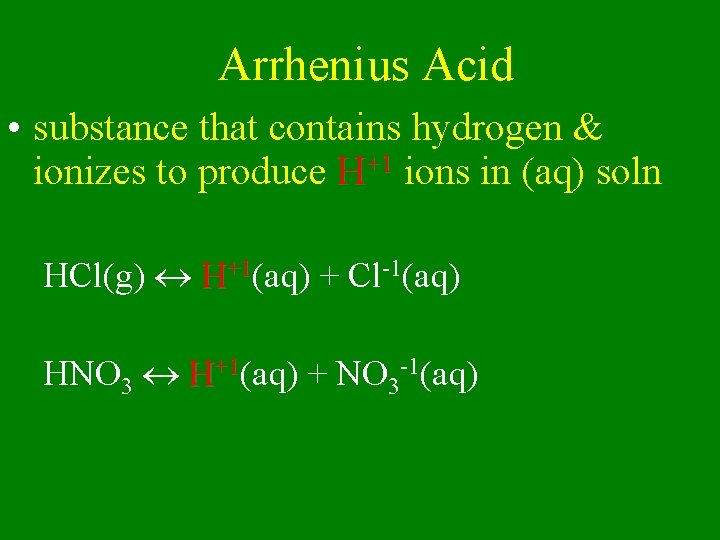
Arrhenius Acid • substance that contains hydrogen & ionizes to produce H+1 ions in (aq) soln HCl(g) H+1(aq) + Cl-1(aq) HNO 3 H+1(aq) + NO 3 -1(aq)
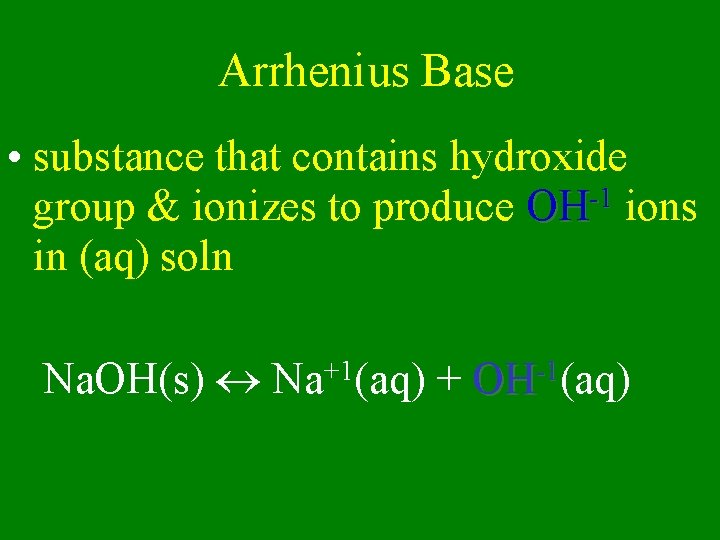
Arrhenius Base • substance that contains hydroxide -1 group & ionizes to produce OH ions in (aq) soln Na. OH(s) Na+1(aq) + OH-1(aq)

Arrhenius Salt • electrolyte where H+1 not only (+) -1 ion and OH not only (-) ion formed in aqueous solution Ex: Na. Cl, Ca. Br 2, KNO 3, NH 4 I
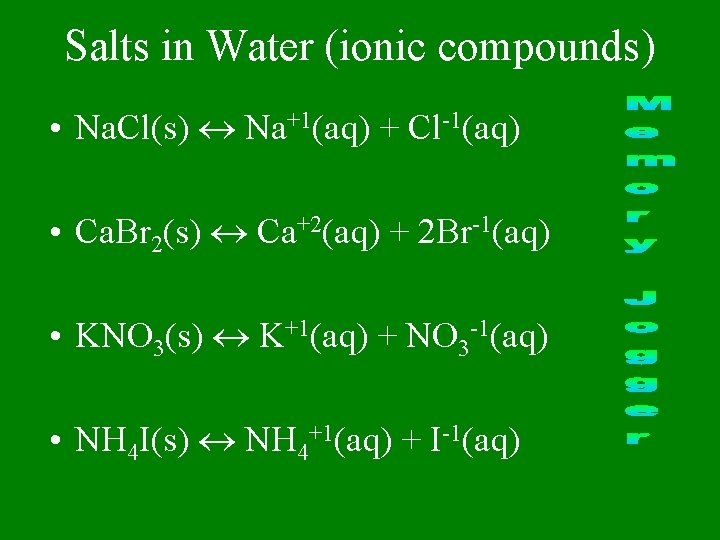
Salts in Water (ionic compounds) • Na. Cl(s) Na+1(aq) + Cl-1(aq) • Ca. Br 2(s) Ca+2(aq) + 2 Br-1(aq) • KNO 3(s) K+1(aq) + NO 3 -1(aq) • NH 4 I(s) NH 4+1(aq) + I-1(aq)
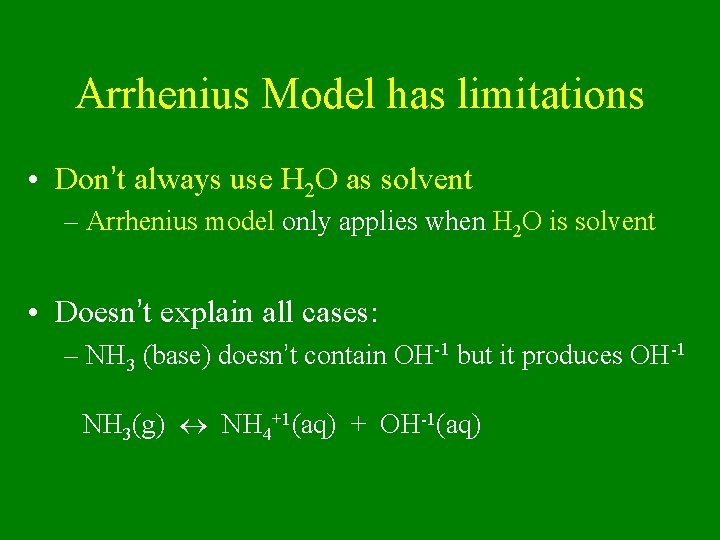
Arrhenius Model has limitations • Don’t always use H 2 O as solvent – Arrhenius model only applies when H 2 O is solvent • Doesn’t explain all cases: – NH 3 (base) doesn’t contain OH-1 but it produces OH-1 NH 3(g) NH 4+1(aq) + OH-1(aq)

Alternate Theory: Bronsted-Lowry • Acid is a proton donor • All Arrhenius acids = Bronsted-Lowry Acids H+1 HX(g) + H 2 O(l) H 3 O+1 + X-1 H+1 forms molecule-ion bond with water molecule H 3 O+1 (hydronium ion)
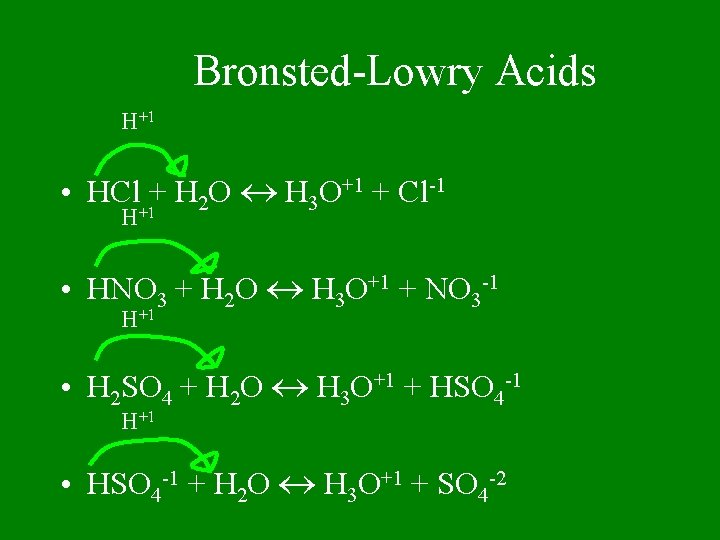
Bronsted-Lowry Acids H+1 • HCl+1+ H 2 O H 3 O+1 + Cl-1 H • HNO 3 + H 2 O H 3 O+1 + NO 3 -1 H+1 • H 2 SO 4 + H 2 O H 3 O+1 + HSO 4 -1 H+1 • HSO 4 -1 + H 2 O H 3 O+1 + SO 4 -2
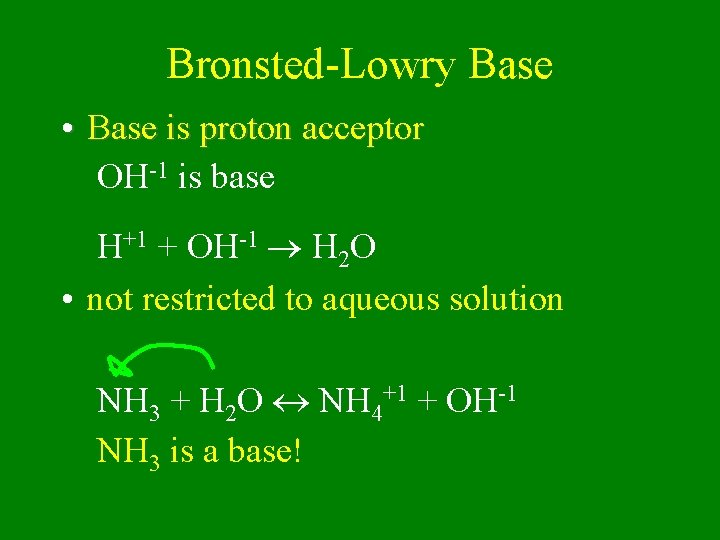
Bronsted-Lowry Base • Base is proton acceptor OH-1 is base H+1 + OH-1 H 2 O • not restricted to aqueous solution NH 3 + H 2 O NH 4+1 + OH-1 NH 3 is a base!
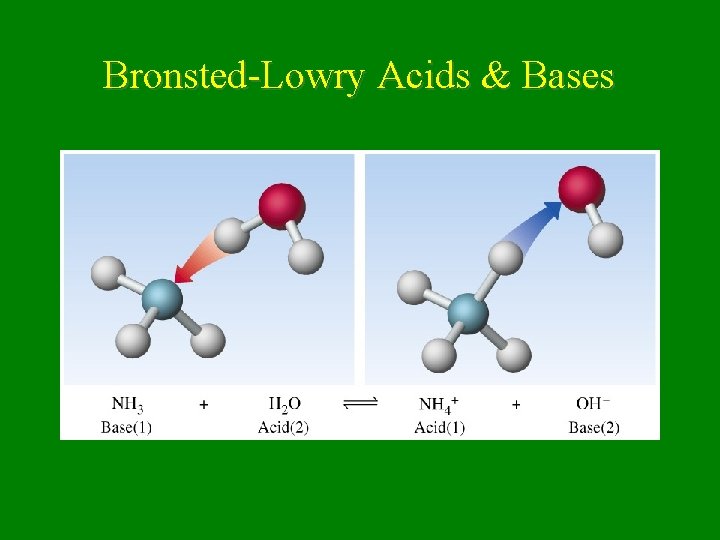
Bronsted-Lowry Acids & Bases
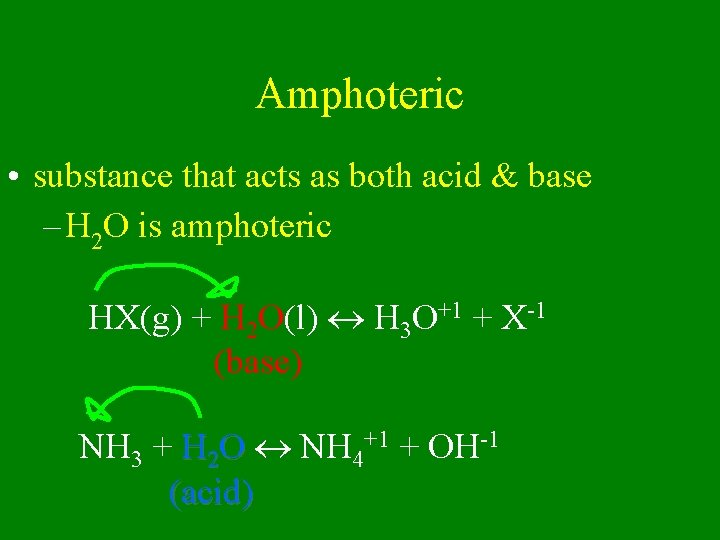
Amphoteric • substance that acts as both acid & base – H 2 O is amphoteric HX(g) + H 2 O(l) H 3 O+1 + X-1 (base) NH 3 + H 2 O NH 4+1 + OH-1 (acid)
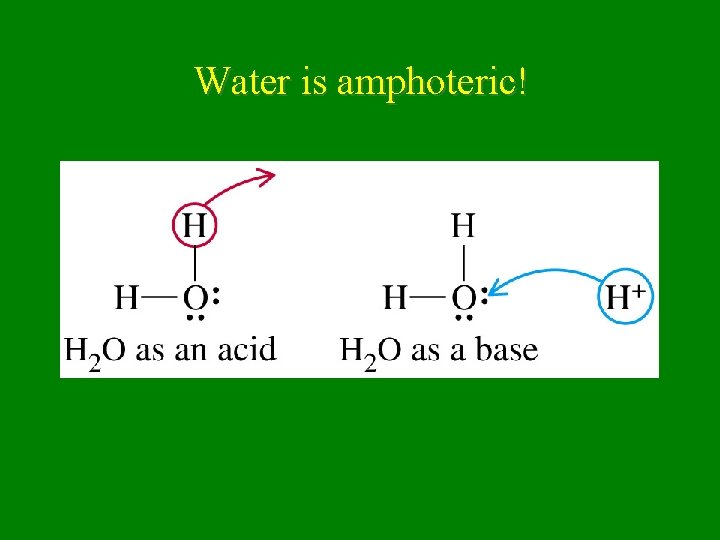
Water is amphoteric!

Naming Acids & Bases
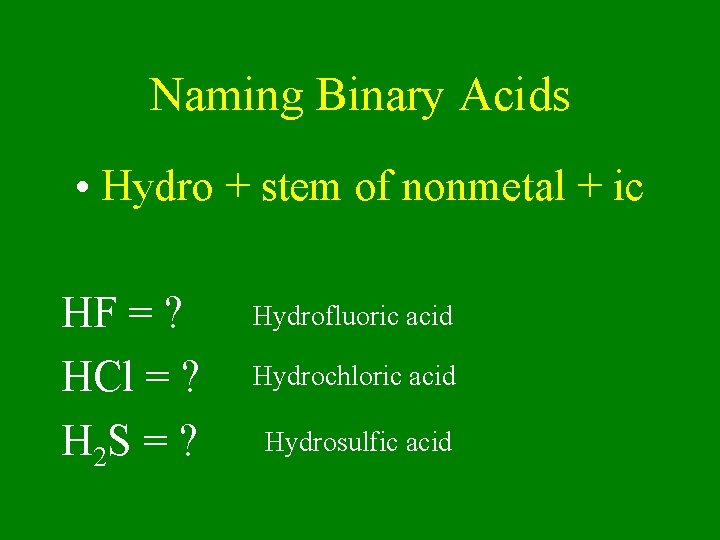
Naming Binary Acids • Hydro + stem of nonmetal + ic HF = ? HCl = ? H 2 S = ? Hydrofluoric acid Hydrochloric acid Hydrosulfic acid

Naming Ternary Acids • Name derived from polyatomic anion (see Table E) • Replace –ite with –ous , add acid HNO 2 is nitrous acid • Replace –ate with –ic, add acid HNO 3 is nitric acid

Ternary Acids • polyatomics with S and P, make stem long again – H 3 PO 4 = phosphoric acid, not phosphic acid – H 2 SO 4 = sulfuric acid, not sulfic acid – H 2 SO 3 = sulfurous acid, not sulfous acid • SEE TABLE K
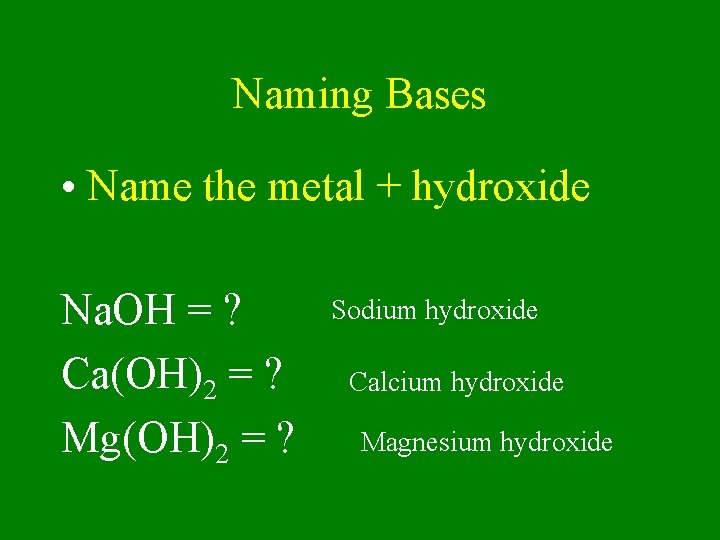
Naming Bases • Name the metal + hydroxide Na. OH = ? Ca(OH)2 = ? Mg(OH)2 = ? Sodium hydroxide Calcium hydroxide Magnesium hydroxide
- Slides: 28