INTRODUCTION TO ACIDS BASES Acids Bases Acids Bases

INTRODUCTION TO ACIDS & BASES Acids & Bases

Acids & Bases Acids and Bases are found in many everyday items, like foods, cleaners, and medicines. Weak acids are strong bases; strong acids are weak bases.

General Properties u sour taste u bitter taste u p. H below 7 u p. H above 7 u react with metals to form H 2 gas u slippery feel u vinegar, milk, soda, apples, citrus fruits u ammonia, lye, antacid, baking soda Chem. ASAP
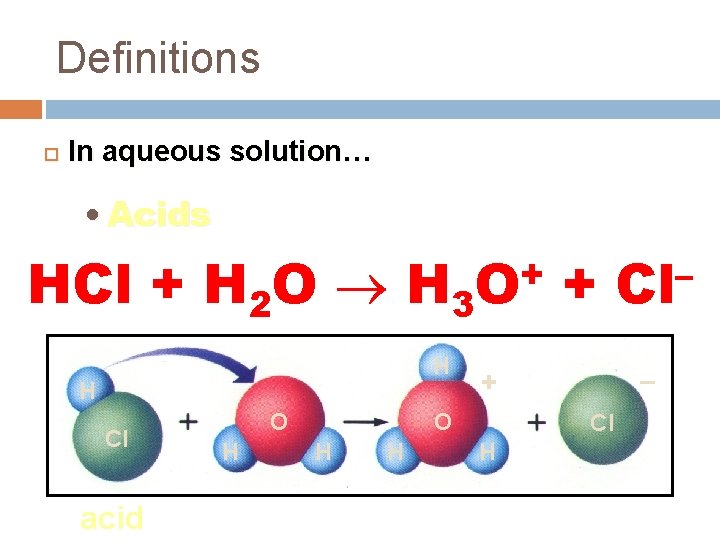
Definitions In aqueous solution… • Acids form hydronium ions (H 3 O+) HCl + H 2 O H 3 H H Cl acid O H + O H – + O H + Cl H – Cl
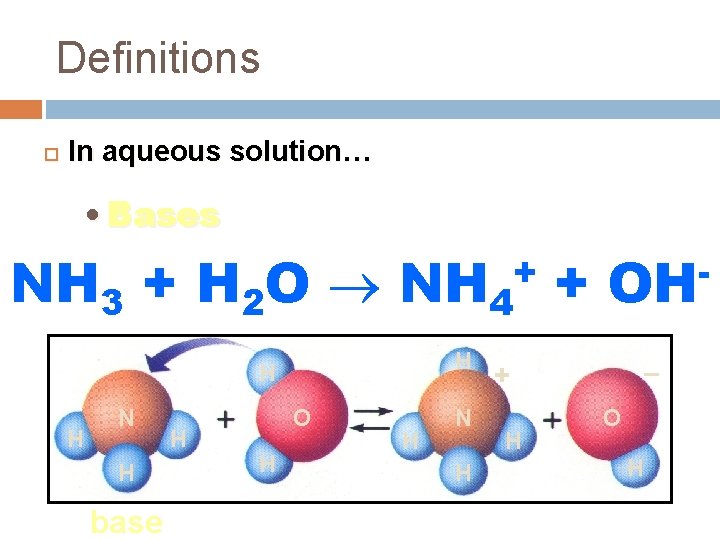
Definitions In aqueous solution… • Bases form hydroxide ions (OH-) NH 3 + H 2 O NH 4 + + H H H N H base H O H H N H OH – + H O H
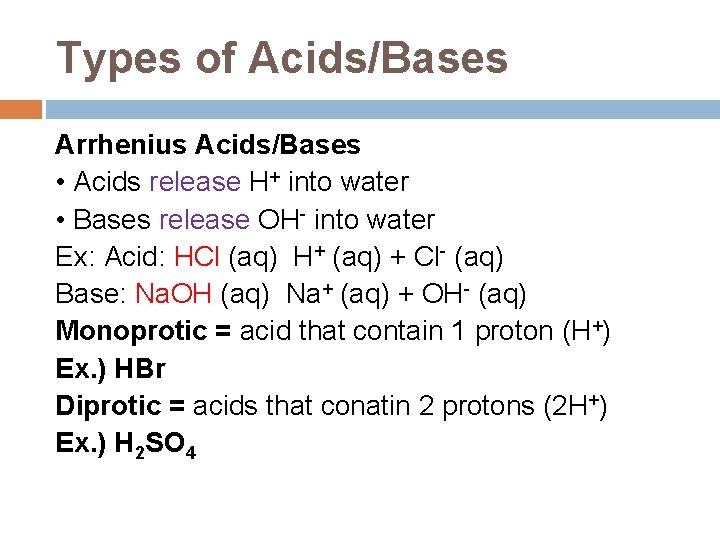
Types of Acids/Bases Arrhenius Acids/Bases • Acids release H+ into water • Bases release OH- into water Ex: Acid: HCl (aq) H+ (aq) + Cl- (aq) Base: Na. OH (aq) Na+ (aq) + OH- (aq) Monoprotic = acid that contain 1 proton (H+) Ex. ) HBr Diprotic = acids that conatin 2 protons (2 H+) Ex. ) H 2 SO 4
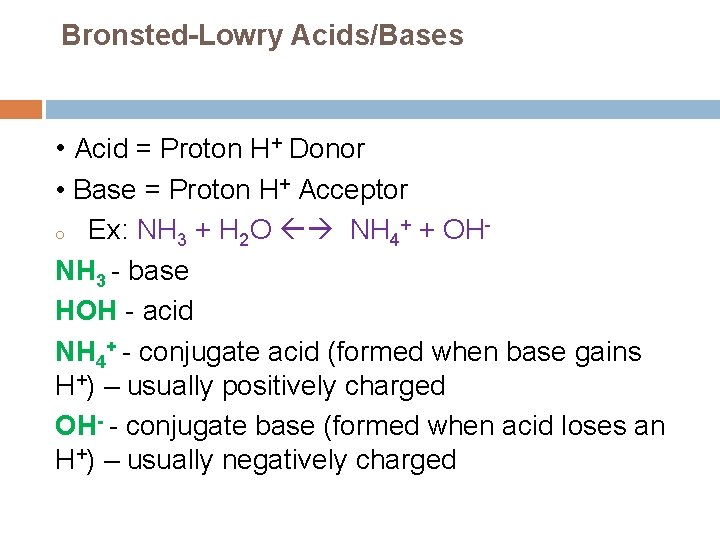
Bronsted-Lowry Acids/Bases • Acid = Proton H+ Donor • Base = Proton H+ Acceptor o Ex: NH 3 + H 2 O NH 4+ + OHNH 3 - base HOH - acid NH 4+ - conjugate acid (formed when base gains H+) – usually positively charged OH- - conjugate base (formed when acid loses an H+) – usually negatively charged

Proton-Transfer Reactions—Direction and Strength The extent to which a proton-transfer reaction takes place depends on the strength of the acids and bases involved in the reaction. Weak acids/bases do not ionize completely, so they tend to re-form in aqueous solution. Therefore, the reactions those compounds undergo with water are reversible. When a strong acid or base reacts with water in aqueous solution, a non-reversible (single-direction) reaction occurs. Example: the strong acid HCl ionizes completely in water due to its high +polarity HCl + H 2 O Cl + once it is broken apart, it does not+reform, hence the single-sided H 3 O arrow pointing to the right. The reason HCl (and all other strong acids) do not re-form is because the conj. bases they make are very weak and have trouble re-gaining the proton from the conj. acid. Therefore, when a strong acid (or base) breaks up, it does not re-form
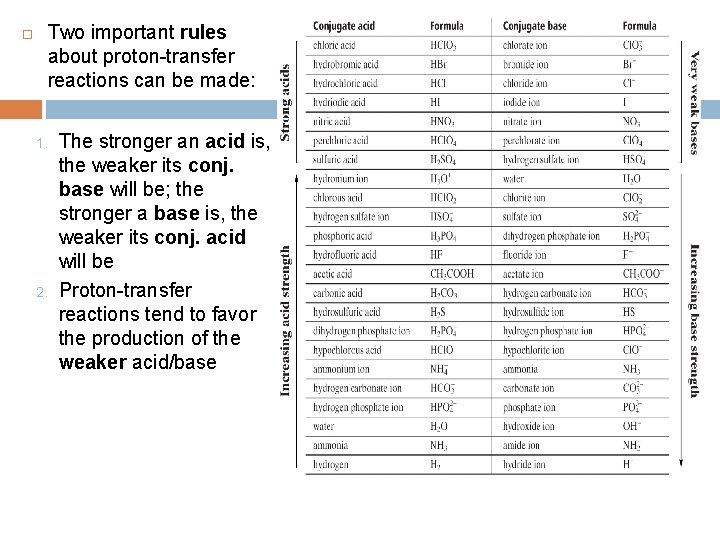
Two important rules about proton-transfer reactions can be made: 1. 2. The stronger an acid is, the weaker its conj. base will be; the stronger a base is, the weaker its conj. acid will be Proton-transfer reactions tend to favor the production of the weaker acid/base

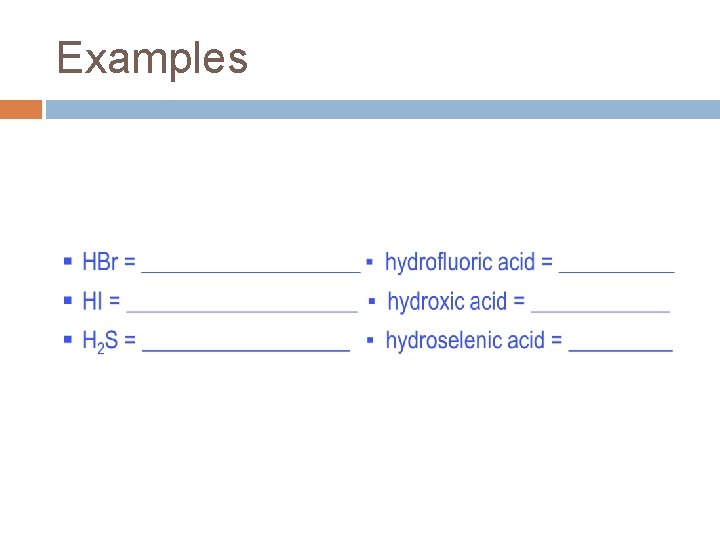
Acid Nomenclature Acids are distinguished from other substances by the presence of one or more hydrogen ions (H+) or hydrogen atoms at the beginning of the formula. To name an acid correctly, you must know the types of acid it is. There are two types of acids: Binary Acids and Oxyacids Binary acid = an acid consisting of only 2 elements (hydrogen + nonmetal). To name a binary acid, follow this procedure: HCl as an 1. Use the prefix “hydro-” example: 2. Name the element the H is bonded to hydro 3. Take the ending off the element name chlorine 4. Add the suffix “-ic” to the element name 5. Add the separate word “acid” to the end hydrochlor- hydrochloric acid
Examples
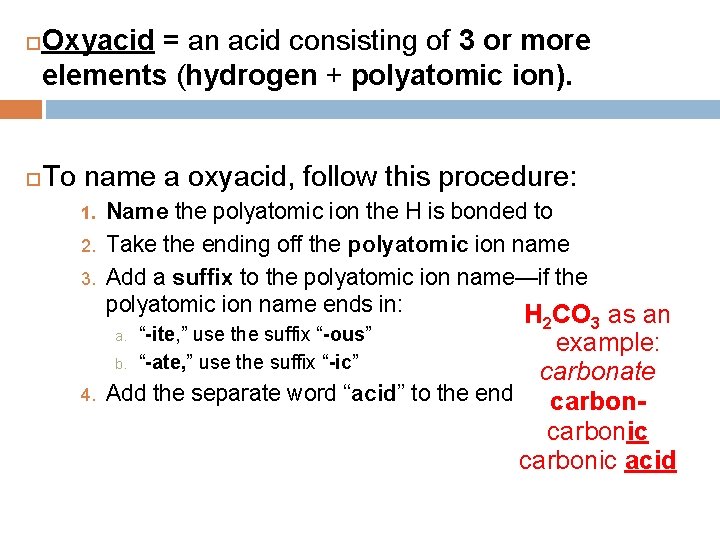
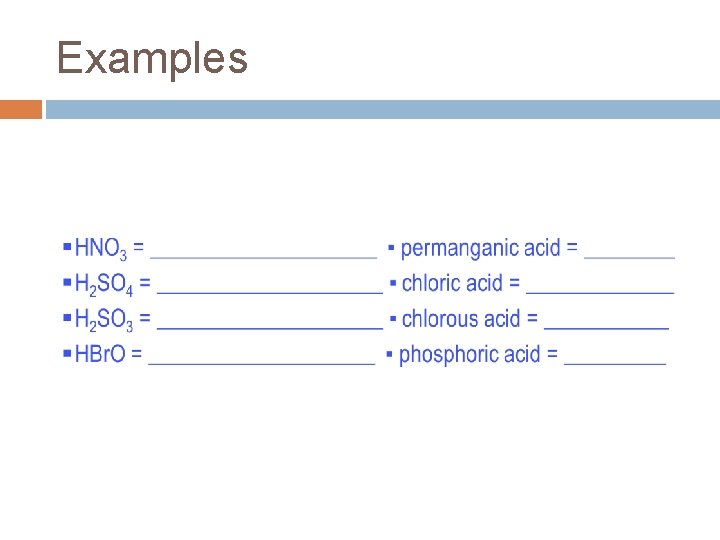
Oxyacid = an acid consisting of 3 or more elements (hydrogen + polyatomic ion). To name a oxyacid, follow this procedure: 1. 2. 3. Name the polyatomic ion the H is bonded to Take the ending off the polyatomic ion name Add a suffix to the polyatomic ion name—if the polyatomic ion name ends in: H 2 CO 3 as an a. 4. “-ite, ” use the suffix “-ous” “-ate, ” use the suffix “-ic” example: b. carbonate Add the separate word “acid” to the end carbonic acid
Examples
- Slides: 13