ACIDS ACIDS BASES BASES SALTS Properties of Acids

ACIDS, ACIDS BASES, BASES & SALTS

Properties of Acids 1. 2. 3. 4. sour taste classified as electrolyte react with bases (neutralization reaction) react with most metals to produce H 2(g) (SR rxn) 5. acids turn litmus paper red
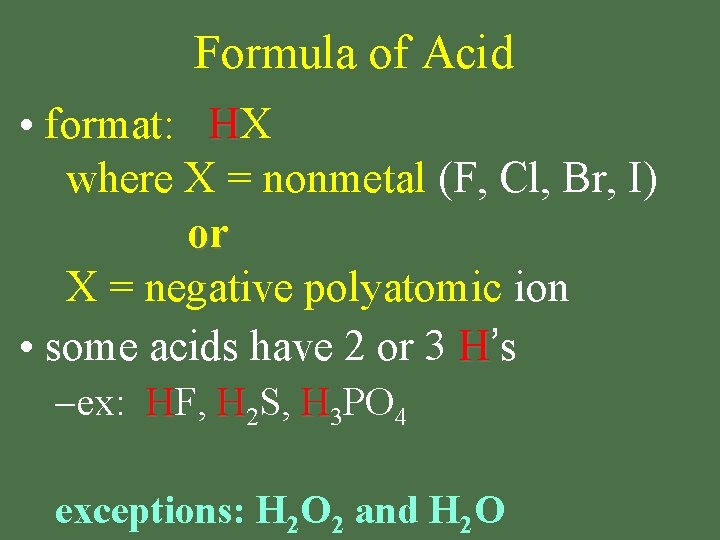
Formula of Acid • format: HX where X = nonmetal (F, Cl, Br, I) or X = negative polyatomic ion • some acids have 2 or 3 H’s – ex: HF, H 2 S, H 3 PO 4 exceptions: H 2 O 2 and H 2 O

Properties of Bases 1. 2. 3. 4. bitter taste slippery or soapy feeling classified as electrolyte react with acids (neutralization rxn) 5. bases turn litmus paper blue
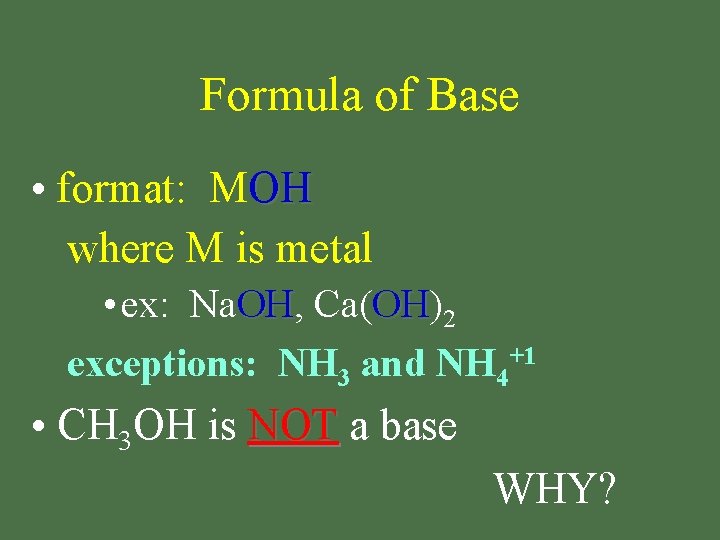
Formula of Base • format: MOH where M is metal • ex: Na. OH, OH Ca(OH) OH 2 exceptions: NH 3 and NH 4+1 • CH 3 OH is NOT a base WHY?

Electrolyte • substance that dissolves in H 2 O to produce aqueous soln that conducts electric current
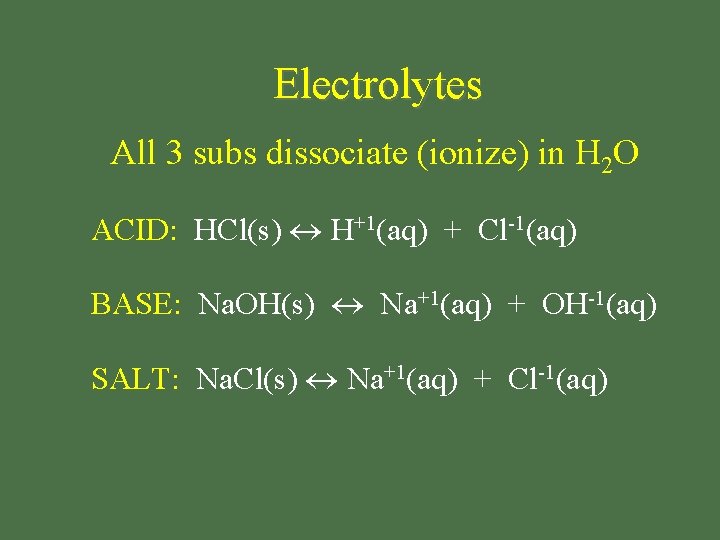
Electrolytes All 3 subs dissociate (ionize) in H 2 O ACID: HCl(s) H+1(aq) + Cl-1(aq) BASE: Na. OH(s) Na+1(aq) + OH-1(aq) SALT: Na. Cl(s) Na+1(aq) + Cl-1(aq)
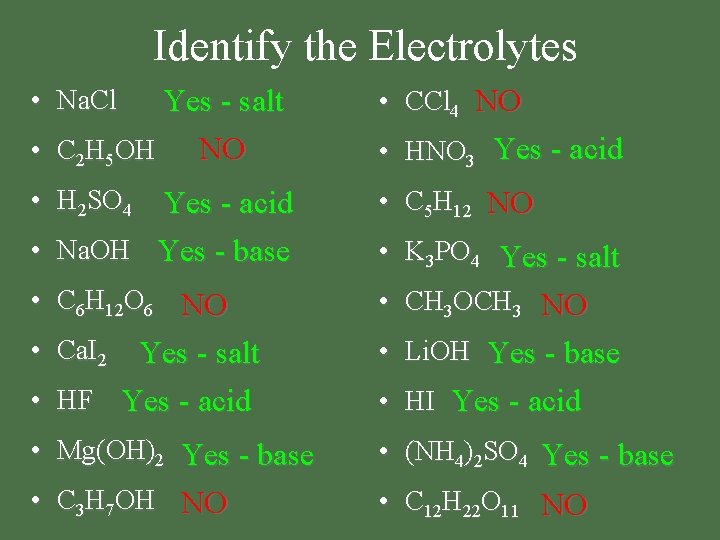
Identify the Electrolytes Yes - salt NO • C 2 H 5 OH • CCl 4 NO • H 2 SO 4 • C 5 H 12 NO • C 6 H 12 O 6 NO • K 3 PO 4 Yes - salt • CH 3 OCH 3 NO • Ca. I 2 Yes - salt • HF Yes - acid • Li. OH Yes - base • Mg(OH)2 Yes - base • (NH 4)2 SO 4 Yes - base • C 3 H 7 OH NO • C 12 H 22 O 11 NO • Na. Cl Yes - acid • Na. OH Yes - base • HNO 3 Yes - acid • HI Yes - acid

Which metals react with acids? • See Table J • All metals above H 2 react with acids • Cu, Ag, and Au do NOT react with acids
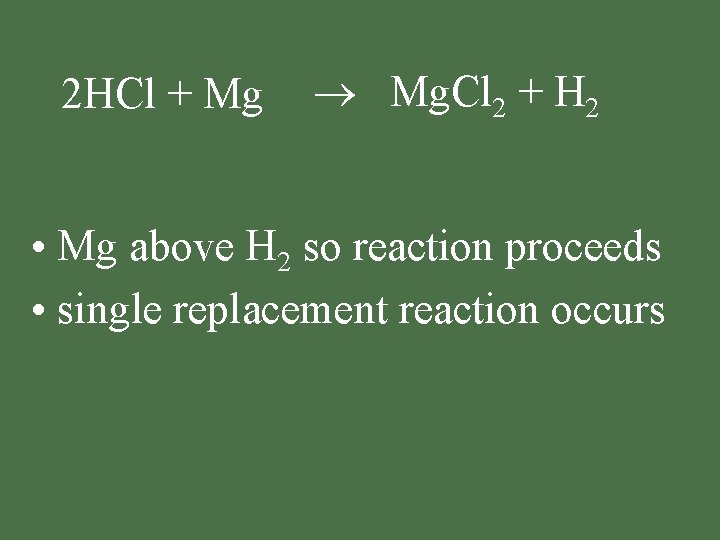
2 HCl + Mg Mg. Cl 2 + H 2 • Mg above H 2 so reaction proceeds • single replacement reaction occurs
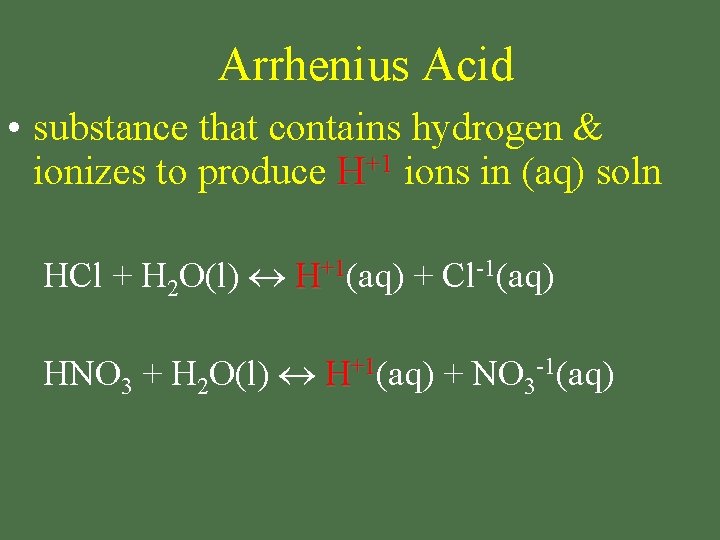
Arrhenius Acid • substance that contains hydrogen & ionizes to produce H+1 ions in (aq) soln HCl + H 2 O(l) H+1(aq) + Cl-1(aq) HNO 3 + H 2 O(l) H+1(aq) + NO 3 -1(aq)
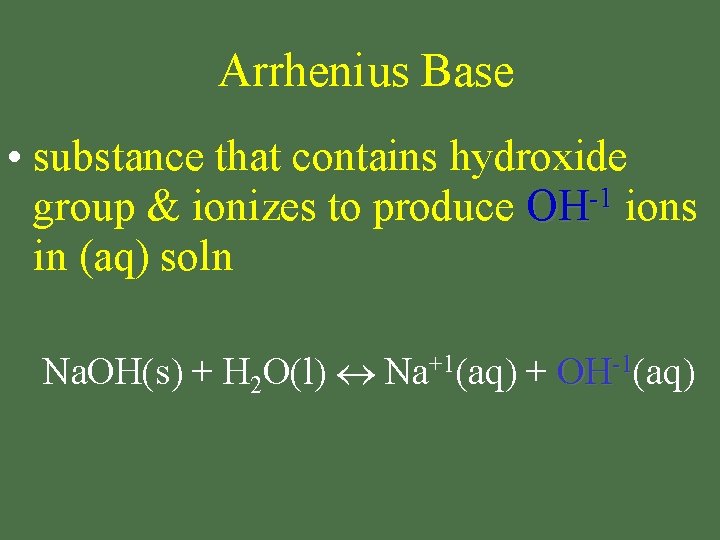
Arrhenius Base • substance that contains hydroxide -1 group & ionizes to produce OH ions in (aq) soln Na. OH(s) + H 2 O(l) Na+1(aq) + OH-1(aq)

Arrhenius Salt • electrolyte where not only (+) ion and OH-1 not only (-) ion formed in aqueous solution ex: +1 H Ca. Br 2(s) Ca+2(aq) + 2 Br-1(aq) KNO 3(s) K+1(aq) + NO 3 -1(aq)
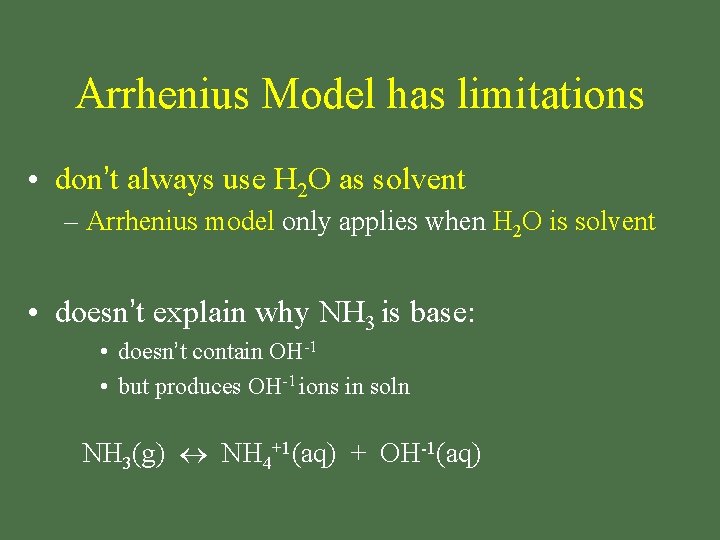
Arrhenius Model has limitations • don’t always use H 2 O as solvent – Arrhenius model only applies when H 2 O is solvent • doesn’t explain why NH 3 is base: • doesn’t contain OH-1 • but produces OH-1 ions in soln NH 3(g) NH 4+1(aq) + OH-1(aq)
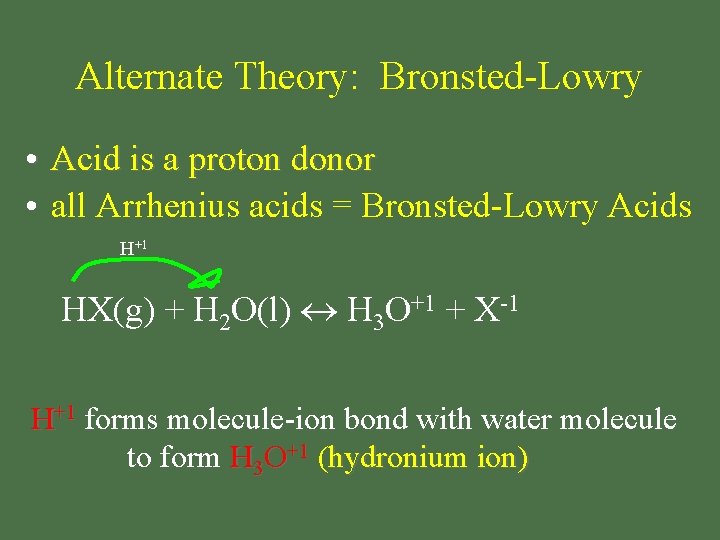
Alternate Theory: Bronsted-Lowry • Acid is a proton donor • all Arrhenius acids = Bronsted-Lowry Acids H+1 HX(g) + H 2 O(l) H 3 O+1 + X-1 H+1 forms molecule-ion bond with water molecule to form H 3 O+1 (hydronium ion)
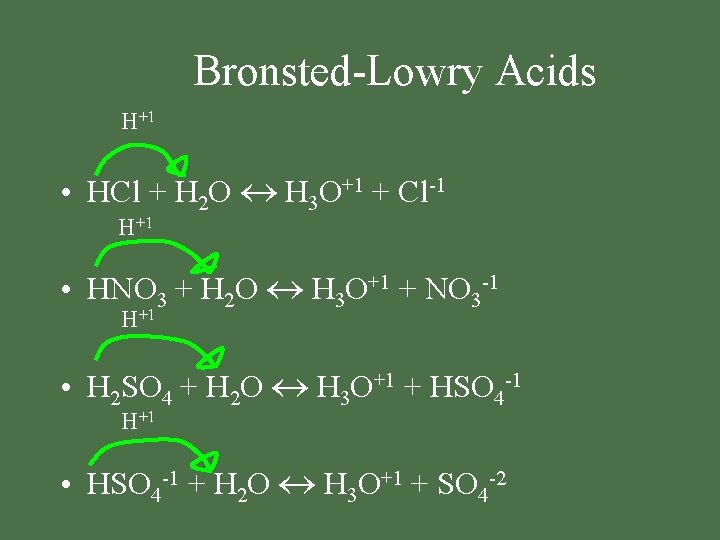
Bronsted-Lowry Acids H+1 • HCl + H 2 O H 3 O+1 + Cl-1 H+1 • HNO 3 + H 2 O H 3 O+1 + NO 3 -1 H+1 • H 2 SO 4 + H 2 O H 3 O+1 + HSO 4 -1 H+1 • HSO 4 -1 + H 2 O H 3 O+1 + SO 4 -2
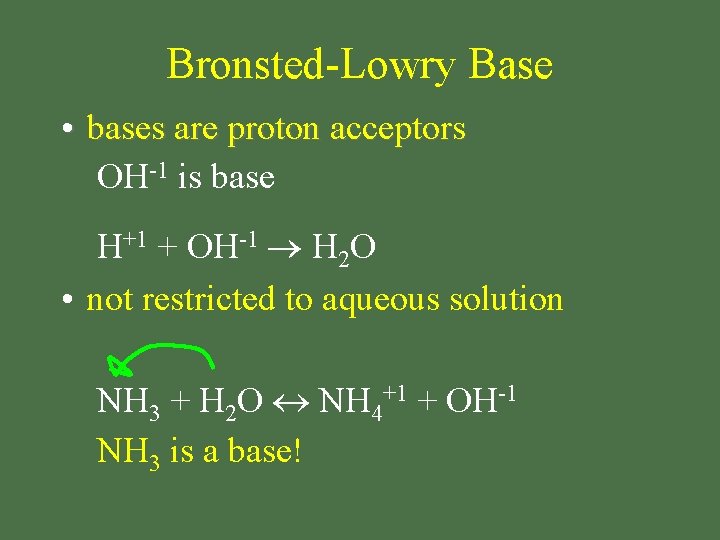
Bronsted-Lowry Base • bases are proton acceptors OH-1 is base H+1 + OH-1 H 2 O • not restricted to aqueous solution NH 3 + H 2 O NH 4+1 + OH-1 NH 3 is a base!
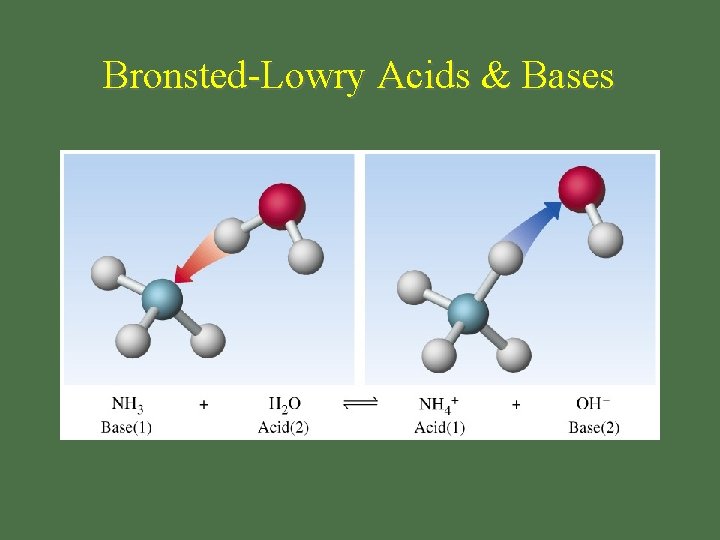
Bronsted-Lowry Acids & Bases

water: self-ionization • H 2 O(l) + H 2 O(l) H 3 O+1(aq) + OH-1(aq) H 3 O+1 = hydronium ion OH-1 = hydroxide ion OR • H 2 O(l) H+1(aq) + OH-1(aq) H+1 : hydrogen ion = proton H+1 & H 3 O+1 used interchangeably
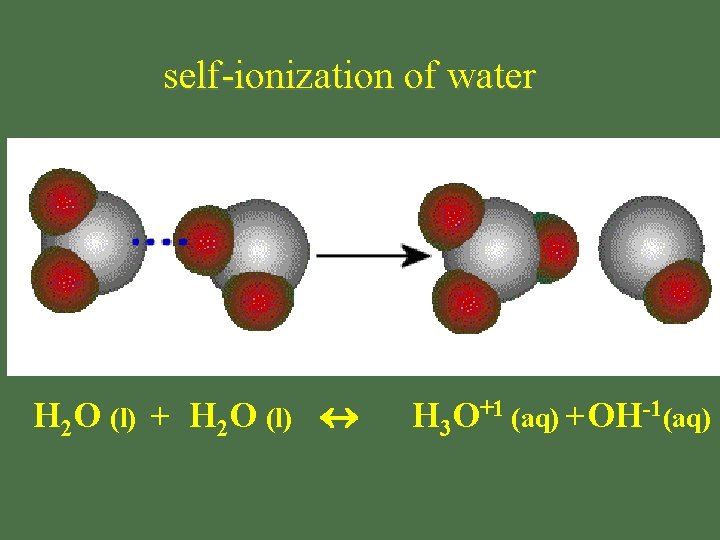
self-ionization of water H 2 O (l) + H 2 O (l) H 3 O+1 (aq) +OH-1(aq)

Acid, Base, or Neutral? • all H 2 O contains some H+1 and some OH-1 ions – pure H 2 O: concentrations very low • neutral solution: [H+1] = [OH-1] • acidic solution: H+1 > OH-1 • basic solution: OH-1 > H+1

Amphoteric • substance that acts as BOTH an acid & a base
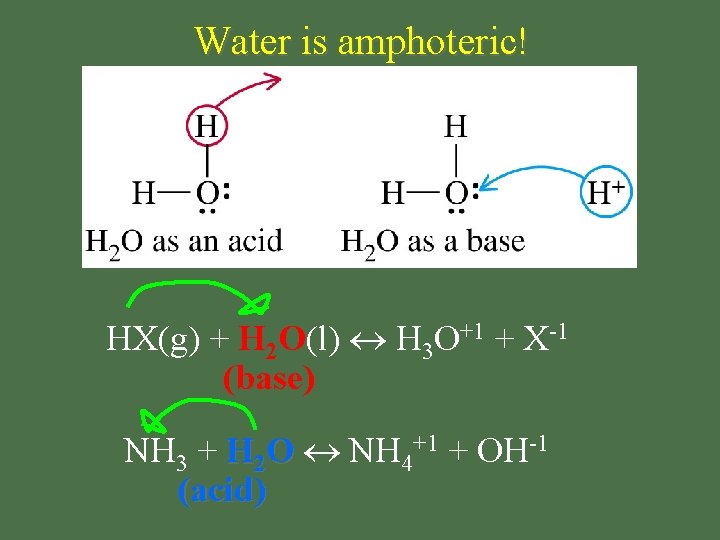
Water is amphoteric! HX(g) + H 2 O(l) H 3 O+1 + X-1 (base) NH 3 + H 2 O NH 4+1 + OH-1 (acid)
- Slides: 23