5 2 Salts Salts are formed when acids

- Slides: 4

5. 2 Salts • Salts are formed when acids and bases react: HCl + Na. OH Na. Cl + H 2 O acid + base salt + water A salt is made up of a positive ion from a base and a negative ion from an acid • Salts are also produced when acids react with oxides (compounds containing oxygen), carbonates or metals. (c) Mc. Graw Hill Ryerson 2007

5. 2 Salts • Table salt, Na. Cl, is found naturally in sea water, salt lakes or rock deposits. w w Salt was once very valuable as a commodity. Iodine is now added to salt to minimize goiter (a disease of the thyroid). Salt is used to cure meat or in pickling Salt is sprinkled on roads and side walks to melt ice. • Na. Cl is only one kind of salt. A salt is made up of a positive ion from a base and a negative ion from an acid Salt crystals in Death Valley w Salts are found in many things: § In batteries, explosives and fertilizers § In multivitamins § In many living cells See pages 234 - 235 (c) Mc. Graw Hill Ryerson 2007

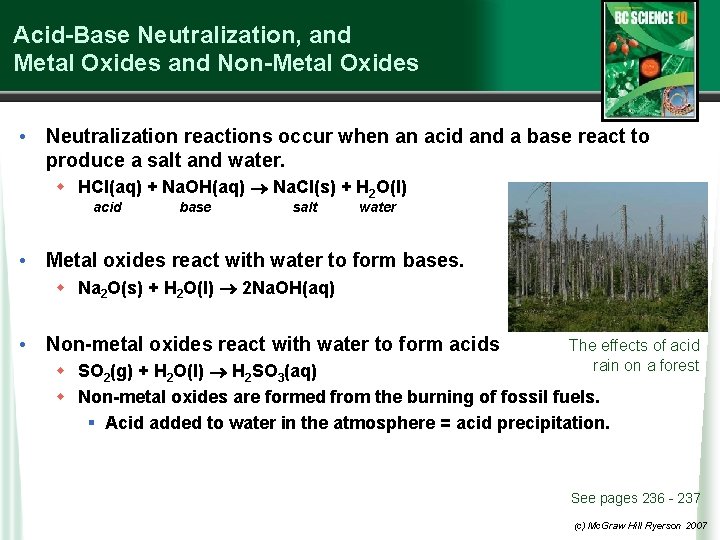
Acid-Base Neutralization, and Metal Oxides and Non-Metal Oxides • Neutralization reactions occur when an acid and a base react to produce a salt and water. w HCl(aq) + Na. OH(aq) Na. Cl(s) + H 2 O(l) acid base salt water • Metal oxides react with water to form bases. w Na 2 O(s) + H 2 O(l) 2 Na. OH(aq) • Non-metal oxides react with water to form acids The effects of acid rain on a forest w SO 2(g) + H 2 O(l) H 2 SO 3(aq) w Non-metal oxides are formed from the burning of fossil fuels. § Acid added to water in the atmosphere = acid precipitation. See pages 236 - 237 (c) Mc. Graw Hill Ryerson 2007

Acids and Metals, and Acids and Carbonates • Acids and Metals w The most reactive metals, at the bottom of groups 1 and 2 on the periodic table, react vigorously with water and acids. w All other metals are less reactive than those in groups 1 and 2. w When metals do react with acids, H 2 gas is usually released. w 2 HCl(aq) + Mg(s) Mg. Cl 2(s) + H 2(g) • Acids and Carbonates w Carbonates neutralize acids, protecting locations with natural carbonate supplies from acid precipitation. w H 2 SO 4(aq) + Ca. CO 3(s) Ca. SO 4(s) + H 2 O(l) + CO 2(g) sulphuric acid calcium carbonate calcium sulphate water dioxide carbon See pages 238 - 239 Take the Section 5. 2 Quiz (c) Mc. Graw Hill Ryerson 2007