A Randomised Phase II study of Accelerated Dose




















- Slides: 27

A Randomised Phase II study of Accelerated, Dose escalated, Sequential Chemo-radiotherapy in Non-Small Cell Lung Cancer Chief Investigator: Professor Matthew Hatton Protocol No: ADSCa. N 2015 Sponsor Ref: GN 12 ON 516 CTU Ref: L 163 INITIATION SLIDES (Version 1, 03 May 2017) Version 1 03 May 2017 1

Study Details § The study is being co-ordinated by Cancer Research UK via the CRUK Clinical Trials Unit, Glasgow § Sponsor of the study is NHS Greater Glasgow & Clyde (legal entity Greater Glasgow Health Board (GGHB)) § Chief Investigator is Professor Matthew Hatton of Weston Park Hospital, Sheffield § Study is being funded by a grant from Cancer Research UK § Study will be conducted according to GCP guidelines § Study conducted in accordance with the EU Directive 2001/20/EC § Study carried out in accordance with the World Medical Association Declaration of Helsinki (1964) and the Tokyo (1975), Venice (1983), Hong Kong (1989), South Africa (1996), Edinburgh (2000), Washington (2002), Tokyo (2004), Seoul (2008) amendments ____________________________________________________________ Please note this presentation has been prepared as part of your site initiation. These slides are a compliment to the protocol; all site study staff must have read and understood the protocol and the study requirements prior to signing off an initiation acknowledgment sheet ___________________________________________________________

Study Team § Chief Investigator : § Co-Investigators: Prof Matthew Hatton (CHART-ED Arm Lead) Dr David Landau (Ideal Arm Lead) Dr Jason Lester (I-START Arm Lead) Prof Corinne Faivre-Finn (Isotoxic IMRT Arm Lead) § Study Statisticians: Jim Paul / Elaine Mc. Cartney § Project Manager: Claire Lawless § Clinical Trial Coordinator: Ann Shaw § Pharmacovigilance: Lindsey Connery / Sinead Traynor § Clinical Trial Monitor: Calum Innes § Radiotherapy Physics: John Fenwick § Radiotherapy QA: Rita Simoes / Nicki Groom § Health Economist: Kathleen Boyd § Consumer Representative: Tom Haswell § Sponsor Representative: Joanne Mc. Garry

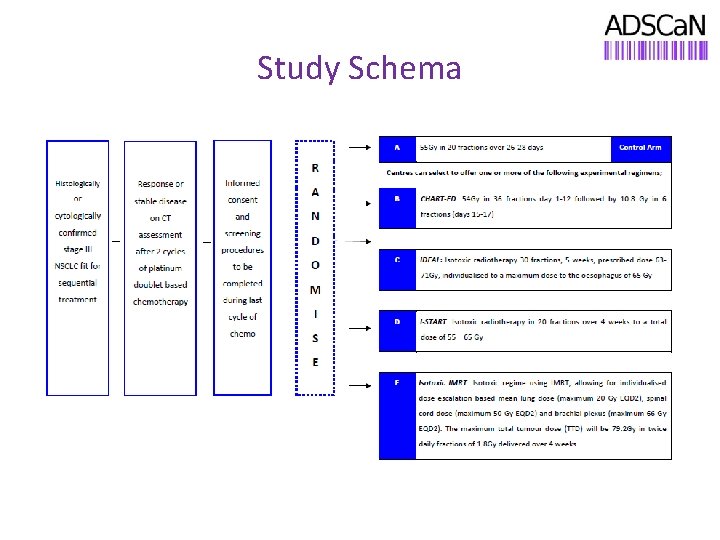
Study Design § We hypothesize that sequential chemo-radiotherapy using accelerated, dose escalated radiotherapy will intensify loco-regional treatment, improve local control and overall survival compared to conventionally fractionated sequential chemo-radiotherapy treatment. This study will take 4 dose escalated accelerated sequential chemo-radiotherapy schedules and compare them with a UK standard sequential chemo-radiotherapy regimen in order to identify the best accelerated regime to take forward into phase III trials. § Randomised phase II screening/”pick-the-winner” design to select a single regimen to go forward for phase III testing. § A novel design will be used that will allow individual centres to randomise to one or more (depending on local resources) of the experimental schedules. In order to maximise the efficiency of the design, sites are encouraged to open as many experimental arms as they can. § The study will enrol 360 patients; 120 patients on the standard arm and 60 on each experimental arm.

Study Schema

Study Endpoints Primary Objective § The primary endpoint is Progression Free Survival (PFS). Secondary Objective(s) § Overall survival § Time to local-regional failure § Toxicity as assessed by NCI CTCAE v 4. 03 § Cost-effectiveness

Inclusion Criteria **Please refer to section 3. 2 and 3. 3 of the current version of study protocol to ensure the correct version of the eligibility criteria are being checked at all times ** Inclusion Criteria § § § § Histologically or cytologically confirmed stage III NSCLC. Performance status (PS) – ECOG 0 -2. Patients with PS 2 can only be included if the local investigator deems the general condition is explained by the primary chemotherapy treatment. Inoperable disease, unsuitable for concurrent chemo-radiotherapy, in the opinion of the treating Oncologist. Patients who have had a complete response, partial response or stable disease on CT assessment after 2 cycles of platinum based chemotherapy. Willing and able to give written informed consent. Aged 16 or over. Adequate PFT results: FEV 1 and/or KCO ≥ 40% of predicted.

Exclusion Criteria § Previous or current malignant disease likely to interfere with the protocol treatment or comparisons. § Medically unstable (unstable diabetes, uncontrolled arterial hypertension, infection, hypercalcaemia, ischaemic heart disease). § Connective tissue disorders (Scleroderma, Systemic Lupus Erythematosus). § Clinically significant interstitial lung disease. § History of physical or psychiatric disorder that would prevent informed consent and compliance with protocol. § Pregnant or lactating women. § Any psychological, familial, sociological or geographical consideration potentially hampering compliance with the trial protocol and follow up schedule. Please note there will be no exception to the eligibility criteria at the time of randomisation. Queries in relation to the eligibility criteria should be addressed via contact with the CTU prior to randomisation. Patients are eligible for the trial if all the inclusion criteria are met and none of the exclusion criteria apply. Version 1 03 May 2017 8

Informed Consent Process Informed consent process: § Two original Consent Forms must be completed by a clinician (or deputy listed on delegation log) § Two originals signed and completed by the patient § Date must be prior to randomisation § Make one photocopy - Original to be filed in Investigator File - Original or photocopy to be given to patient (+PIS) - Photocopy to be filed in hospital notes § Consent Form must not be sent to your coordinating trials office § New version of Patient Information Sheet must be provided to patients consented with previous version. CONSENT WITHDRAWAL Consent Withdrawal occurs if a patient specifically asks to withdraw their consent at any point in the study. If this occurs: § § Document clearly in the patient notes that the patient has withdrawn consent, the level of consent withdrawal and the reason (if the patient has given any) Contact CRUK CTU to advise of consent withdrawal. A Consent Withdrawal Notification form will be provided to site. Complete form and return to the CRUK CTU. No further follow-up should be collected on the patient from that point onwards. Version 1 03 May 2017 9

Randomisation Process • Check that patient fulfils all eligibility criteria as per study protocol • Complete Randomisation Form • Site staff must contact the CRUK Clinical Trials Unit, Glasgow to randomise the patient. Randomisation can be performed by either telephone or fax on the following numbers: Tel no: 0141 301 7952 Fax no: 0141 301 7946* 08. 30 -17. 00 Monday - Thursday 08. 30 -16. 30 Friday, except public holidays * Faxes received outside of office hours will be processed the next working day • Each patient randomised will be allocated a unique 3 digit sequential patient ID number for the trial Version 1 03 May 2017 10

Study Treatment Patients who are eligible for this study will receive 2 -4 cycles of platinum doublet neo-adjuvant chemotherapy. The recommended non-platinum agents are pemetrexed or vinorelbine for non-squamous carcinoma and gemcitabine or vinorelbine for squamous carcinoma. If patients fulfil all eligibility criteria they will then be randomised to receive: Arm A: STANDARD: Arm B: CHART-ED: 55 Gy in 20 fractions over 28 days. Arm C: IDEAL: Isotoxic radiotherapy 30 fractions, 5 weeks, prescribed dose 6371 Gy, individualised to a maximum dose to 1 cc of the oesophagus of 65 Gy and defined constraints for lung, heart and brachial plexus. Fractionation is once a day for 4 days a week and twice daily on 1 day a week. Arm D: I-START: Isotoxic radiotherapy 20 fractions, 4 weeks total dose of 55 – 65 Gy. 54 Gy, 36 fractions, 12 days then 10. 8 Gy, 6 fractions (day 15 -17).

RECIST Reporting Requirements Disease evaluation will be performed according to RECIST 1. 1. (Please go to the following website to access RECIST Version 1. 1, January 2009: http: //www. eortc. be/recist/documents/RECISTGuidelines. pdf ) § § All patients will undergo baseline evaluation tumour assessment with a CT thorax and upper abdomen. The same imaging modalities should be used for the duration of the trial. All radiological investigations must be reported as per protocol / RECIST version 1. 1. § Source documentation of this must be available for review if the original report has had to be supplemented to bring it in line with protocol requirements § CRUK CTU, Glasgow have produced a worksheet to assist with the documentation of study specific reporting and will make this available to any participating site upon request to the study monitor Version 1 03 May 2017 12

Process for notification of protocol deviations by sites § All participating sites must notify the Sponsor (via CRUK CTU) of all deviations from the protocol or GCP immediately § The Sponsor requires a report on the incident(s) and a protocol deviation form will be provided during site initiation which should be used for informing of protocol deviations § If site staff are unsure whether a certain occurrence constitutes a deviation from the protocol or GCP, the CRUK CTU trial team and Sponsor can be contacted immediately to discuss. The Sponsor will assess all incidents with respect to the criteria of a “serious breach” Version 1 03 May 2017 13

Management of Serious Breaches § The PI and site staff will be notified of any potential issues that have been identified which are considered to require escalation to the sponsor. § The CRUK CTU will act as the liaison between the PI and Sponsor to clarify any details or request any further information in relation to the issues. § Once agreed by the Sponsor that the issues are a potential serious breach or are a serious breach they will prepare the report to the REC. § It is important that sites respond to requests for further information in a timely manner as serious breaches are required to be reported within 7 days of the sponsor becoming aware of issue. Version 1 03 May 2017 14

Monitoring (1) CENTRAL MONITORING Study sites will be monitored centrally by checking incoming forms for compliance with the protocol, data consistency, missing data and timing. Study staff will be in regular contact with site personnel (by phone/fax/email/letter) to check on progress and deal with any queries that they may have. Monitoring Visits/Schedule: § Participating study sites will be monitored by both telephone and on-site monitoring visits. § Each site will receive two telephone monitoring calls and a minimum of one on site visit during the course of the trial. § The CRUK CTU reserves the right to undertake a for-cause monitoring visit should this be considered necessary at any stage. Version 1 03 May 2017 15

Monitoring (2) Telephone & Remote Monitoring: § The time & date will be agreed with a member of the Site Study Team. § A pro forma covering the questions which will be covered during the telephone monitoring visit will be sent with confirmation of the agreed date. § Please set aside 50 to 70 minutes for this call. On Site Monitoring: § All patient source documentation should be made available to enable Source Document Verification by the Clinical Trial Monitor. § A full working day is required for on-site visits & arrangements should be in place to facilitate the monitor access on the agreed date. § If sites are able to provide printed results/reports these must be filed in the source documents. § If a site is using electronic data reporting systems or electronic records & hard copies are not available – the clinical trial monitor must be permitted access to the system either by being issued with a temporary login or a member of staff available for the duration of the visit to facilitate electronic access to authorised reports/results. § All findings will be discussed at an end of visit meeting and any unresolved issues raised as Action Points. § Action Points will be followed up by the monitor until resolved. Version 1 03 May 2017 16

Data Management The trial uses the electronic data capture system MACRO and paper randomisation forms. A user guide will be provided and site staff access will be provided once the site is activated to recruitment. PAPER CRFs FOR THE TRIAL: • • Randomisation Form Pregnancy Notification Form SAE Form CRF Completion Guidelines will be provided. ELECTRONIC CRF VISITS: • • • Baseline Weekly Treatment and Treatment Summary Follow Up and Tumour Assessment Pregnancy Notification Consent Withdrawal Notification CRF/ e. CRF COMPLETION TIMELINES: • • Data entry – within 6 weeks of the patient visit Resolution of queries – within 6 weeks of receipt Central Review of Data CRUK CTU will regularly review the data for compliance with the protocol, and for inconsistent or missing data. Should any missing data or data anomalies be found within the e. CRFs upon CTU review, queries will be generated within the MACRO ® study database for the site to access and resolve. Sites are expected to review and respond to queries within the database in a timeous manner. Any issues identified at sites in relation to poor data/slow response to data queries will be managed as per the data escalation process. To minimise the number of data queries being sent to sites, the CRUK CTU will develop a data rulings document and resolve as many self-evident queries as possible in-house.

Data Management (2) DATA ESCALATION PROCESS § CRUK CTU will regularly chase outstanding data from participating sites. Routine requests for outstanding data and data queries will be performed quarterly or more regularly if required. § Sites will be routinely requested to complete outstanding data and data queries within 6 weeks of receiving the queries or the e. CRF being due for completion. § Trigger reports will be run quarterly at the same point as the routine requests for data. If 20% of forms are overdue for more than 3 months (at least 10 forms meeting this criteria) or any forms greater than 6 months overdue the site will be contacted. A log will be kept of any sites meeting a trigger point. § If a site consistently meets a trigger point an escalation process will begin. See the protocol for further information. Version 1 03 May 2017 18

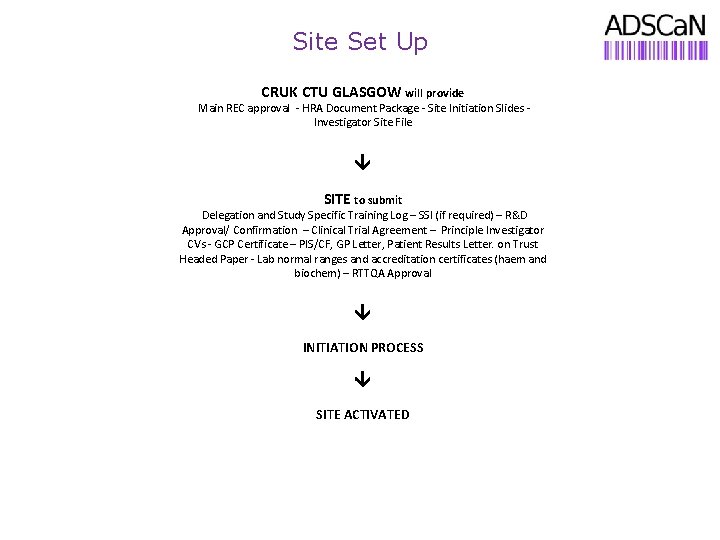
Site Set Up CRUK CTU GLASGOW will provide Main REC approval - HRA Document Package - Site Initiation Slides - Investigator Site File SITE to submit Delegation and Study Specific Training Log – SSI (if required) – R&D Approval/ Confirmation – Clinical Trial Agreement – Principle Investigator CVs - GCP Certificate – PIS/CF, GP Letter, Patient Results Letter. on Trust Headed Paper - Lab normal ranges and accreditation certificates (haem and biochem) – RTTQA Approval INITIATION PROCESS SITE ACTIVATED

Confidentiality § All information collected during the course of the study will be kept strictly confidential. Information will be held securely on paper and electronically at the CRUK CTU. The CRUK CTU will comply with all aspects of the 1998 Data Protection Act and National Health Service Guidelines for storage, transmittal and disclosure of patient information. § Data on patients treated on the study will be held in study case report forms (CRFs), these files will be identified by a trial number and patient initials only. § Patient identifiable data (such as full name/or initials with date of birth) should not be sent on email correspondence. If you need to refer to a patient use trial and patient number. § Where central monitoring of source documents by CRUK CTU (or copies of source documents) are required (e. g. scan results of blood results) the personal data of the patients must anonymised on the report e. g. black out the patient’s name and any other identifiable information. § Where anonymisation of documentation is required, sites are responsible for ensuring no patient identifiable data is present before sending to CRUK CTU. Version 1 03 May 2017 20

Record Retention and Archiving § § Archiving of the study essential documents should be performed by both the participating trial site and Sponsor/CRUK CTU. Participating sites are responsible for archiving their trial related documentation and should follow the requirements of their R&D Office in conjunction with advice from the CRUK CTU and Sponsor regarding the duration of document retention. § Sites should not archive their study documentation until they have been instructed by the CRUK CTU or Sponsor that they are able to do so. Where possible, at the time of archiving, sites will be notified of the archiving retention period. If this is not confirmed at the time of archiving, sites should not destroy archived documentation until authorisation is given from the Sponsor. § The Sponsor and CRUK CTU will be responsible for archiving the Trial Master File (TMF) and all other essential trial documentation that is not held at participating trial sites as per their applicable SOPs. Version 1 03 May 2017 21

Safety Reporting Safety reporting, notification and expedited reporting procedures will adhere to the National Research Ethics Service (NRES) guidance set out for safety reporting in any research other than CTIMPs (Safety Reports for all Other Research). Adverse Events (AEs) § AEs will be recorded and managed in accordance with the study protocol. Serious Adverse Events (SAEs) A serious adverse event (SAE) is defined as an untoward occurrence considered related to the study radiotherapy that: a) b) c) d) e) f) results in death within 90 days of last dose of radiotherapy. is life-threatening. requires hospitalisation or prolongation of existing hospitalisation. results in persistent or significant disability or incapacity. consists of a congenital anomaly or birth defect or is otherwise considered medically significant by the investigator. * Only events meeting the above definition of a SAE and are considered to be related to study radiotherapy are to be reported as SAEs. § Serious Adverse Events (SAEs) must be reported to the Pharmacovigilance Office, CRUK CTU, Glasgow immediately and under no circumstances should this exceed 24 hours. § SAEs are required to be reported from the start of radiotherapy treatment up to an including 30 days after the last fraction of radiotherapy. Any SAE that occurs after 30 days post radiotherapy (with no time limit) is also required to be reported if the Investigator thinks the SAE is related to protocol treatment, is not listed as expected and is medically important **For full details on Safety Reporting please section 7 of the current study protocol** 22

Safety Reporting (2) SAE References § For each SAE received for a patient a SAE reference is allocated by CRUK CTU Pharmacovigilance team (for e. g. -1 for first event and -2 for patients second event ) where details of SAE are recorded on the CRF for e. g. Adverse Event table the SAE reference number allocated to the event should be recorded on the CRF. Pharmacovigilance (PV) Data Escalation Process § The CRUK CTU Pharmacovigilance team will regularly chase outstanding data from participating sites in relation to SAE report forms with request for data/queries to be returned within a required timeframe. § If following requests a response is not received from site staff an escalation process will begin. CRUK CTU Pharmacovigilance Team § If you have any queries they should contact the CRUK CTU Pharmacovigilance Team who will be happy to provide advice. Email: mvls-ctu-pv@glasgow. ac. uk Telephone number: 0141 301 7945/7211/7953 Version 1 03 May 2017 23

Radiotherapy/Radiotherapy QA ** Please refer to the current version of the Radiotherapy Planning and Delivery Guidelines for full details on radiotherapy requirements ** Radiotherapy Quality Assurance § The radiotherapy quality assurance (RT QA) programme for the study will be designed and implemented by the National Radiotherapy Trials QA (RTTQA) Group Pre-Study QA: § Facility Questionnaire § Outlining Benchmark Cases § Planning Benchmark Case § Process document Ongoing study requirements: § Anonymised CT images and RT plans for all patients recruited must be exported to Mount Vernon National RTTQA Group. Radiotherapy plans will be subject to retrospective review by the Mount Vernon National RTTQA Group to ensure adherence to the trial RT Planning and Delivery Guidelines. Non-compliance will be highlighted to the CI, local PIs and the Trial Management Group by the Mount Vernon National RTTQA Group and a decision will be made regarding further action for that patient and future patients recruited at that site. Version 1 03 May 2017 24

Translational Research Requirements Blood samples § A blood sample for research purposes is collected at the Baseline visit. Samples will be processed and stored on site, until being batch shipped to the Northern Ireland Biobank (NIB) Archival Tissue Samples § Patients are asked to consent to FFPE archival tumour block being provided. Sites will be requested to provide this sample retrospectively. At such time, CRUK CTU will contact sites to begin collection of these samples. Pre paid Royal Mail safe boxes will be provided to sites for postage of these samples. Full arrangements will be provided to sites at the time of collection of samples. Consent § Consent for translational research is optional. If patients do not consent to providing blood or tissue samples, it will not affect their care and they will still be eligible to participate in the ADSCa. N Study **For full details on handling, processing and shipment of these samples please refer to the current study Lab Manual** Version 1 03 May 2017 25

Other Staff The Principal Investigator has overall responsibility for the conduct of the clinical trial at the trial site. BUT § All staff must comply with GCP. § Staff should only perform tasks delegated to them. § Staff should ensure that their details are available to the Investigator. § Staff should maintain appropriate confidentiality at all times Version 1 03 May 2017 26

Contact Details for CRUK CTU Glasgow Project Manager Clinical Trial Coordinator Claire Lawless Ann Shaw Tel: 0141 301 7947 Tel: 0141 301 7215 E-mail: claire. lawless@glasgow. ac. uk E-mail: ann. peek@glasgow. ac. uk Pharmacovigilance Manager Pharmacovigilance CTC Lindsey Connery Sinead Traynor Tel: 0141 211 0352 Tel: 0141 211 3968 Fax: 0141 301 7213 Fax: 0141 301 7213 E-mail: lindsey. connery@glasgow. ac. uk E-mail: sinead. traynor@glasgow. ac. uk Clinical Trial Monitor Postal Address Calum Innes Cancer Research UK Clinical Trials Unit Tel: 0141 211 3801 Level 0, Mobile : 07825 030 429 Beatson West of Scotland Cancer Centre E-mail: calum. innes@glasgow. ac. uk 1053 Great Western Road Glasgow, G 12 0 YN Version 1 03 May 2017 27