PLUTO U 185 A randomised phase II study







































- Slides: 54

PLUTO (U 185) A randomised phase II study investigating Pazopanib versus weekly Paclitaxel in relapsed or progressive transitional cell carcinoma (TCC) of the urothelium Eudra. CT Number: 2011 -001841 -34 ISCRTN 73030316 INITIATION SLIDES (VERSION 4: 11 th October 2013)

PLUTO - Study Details • The study is part of the NCRN/Glaxo. Smith. Kline collaboration • The study is being co-ordinated via the Cancer Research UK Clinical Trials Unit, Glasgow. • Sponsor of the study is Greater Glasgow Health Board (GGHB) and University of Glasgow (GU) • Joint Chief Investigators are Dr Rob Jones (Beatson West of Scotland Cancer Centre) and Dr Tom Powles (St. Bart’s Hospital, London) • Study is being funded by an educational grant from Glaxo. Smith. Kline and is endorsed by CTAAC (Cancer Research U. K. )

PLUTO - Study Team • Joint Chief Investigator : Dr Robert Jones • Joint Chief Investigator: Dr Tom Powles • Study Statistician: Jim Paul / Caroline Bray • Project Manager: Judith Dixon • Pharmacovigilance: Lindsey Connery • Clinical Trial Coordinator: Anna Morris • Clinical Trial Monitor: Eileen Smillie

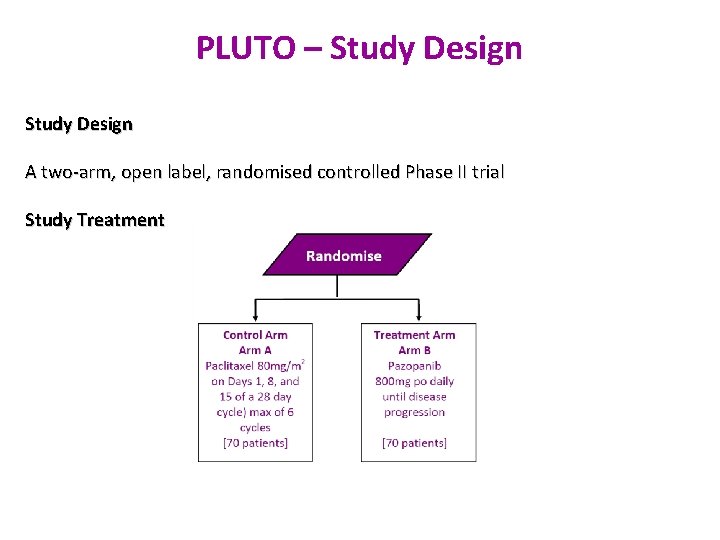
PLUTO – Study Design A two-arm, open label, randomised controlled Phase II trial Study Treatment

PLUTO – Population and Aims Study population • 140 patients with progressive disease during or after one prior platinum-based chemotherapy regimen for advanced disease or as peri-operative therapy for muscle-invasive / node positive disease (if completed < 12 months prior to documented disease progression). The regimen must have included either cisplatin or carboplatin • Study Aims • Primary Objective: To provide preliminary evidence on whethere is a survival advantage for Pazopanib compared to weekly Paclitaxel as second line treatment for advanced urothelial cancer • • Secondary Objectives: To estimate the difference in progression-free survival To assess clinical benefit after 12 and 24 weeks of treatment To observe the safety, toxicity and Quality of Life (Qo. L) of both treatments

PLUTO – Study Eligibility Inclusion Criteria: • • • Histologically or cytologically confirmed TCC (bladder, renal pelvis, ureter, urethra) which is locally advanced or metastatic (T 4 b and/or N 1 -3 and/or M 1). Patients with mixed or differentiation pattern pathology will be permitted entry providing that TCC is a component pathology Progressive disease during or after one prior course of platinum based chemotherapy for advanced disease or as peri-operative therapy for muscle invasive/node positive disease (if completed <12 months prior to documented disease progression). The course of chemotherapy must have included either Cisplatin and/or Carboplatin. Patients who have had two courses of platinum containing chemotherapy are eligible if one of these was given peri-operatively, and provided that there was a chemotherapy free interval of at least 12 months between completing the first course and commencing the second course of chemotherapy. Chemotherapy given during radical radiotherapy as a radiosensitizer will not be considered as chemotherapy treatment for the purposes of study eligibility Age ≥ 18 years Measurable disease by RECIST 1. 1 Adequate organ function (see protocol for details) Signed and dated informed consent Negative pregnancy test for women of childbearing potential Life expectancy of 3 months or more ECOG 0, 1 or 2

PLUTO – Study Eligibility Exclusion Criteria: • Congestive heart failure, myocardial infarction, coronary artery bypass graft or thrombotic cerebrovascular event in the previous 6 months, or ongoing severe or unstable arrhythmia requiring medication • History of clinically significant bleeding in the 6 months prior to study initiation • Major surgery or trauma within 28 days prior to first dose of investigational product and/or presence of any unhealed wound, fracture or ulcer • Cerebrovascular accident including transient ischemic attack, pulmonary embolism or DVT within the past 6 months • History of another malignancy in the last 5 years • Ongoing major gastrointestinal disease including unstable inflammatory bowel disease or bleeding peptic ulcer • Known endobronchial lesions which have a high risk of pulmonary haemorrhage • Previously identified brain or CNS metastases at baseline (see protocol for exceptions) • Pregnant or breastfeeding

PLUTO – Study Eligibility Exclusion Criteria continued: • Administration of any investigational drug within 28 days or 5 half lives prior to receiving the first dose of study treatment • Radiation therapy, surgery or tumour embolisation within 14 days of study drug • Chemotherapy, immunotherapy, biological therapy or investigational therapy within 28 days of study treatment • Peripheral neuropathy of grade 2 or more • Any ongoing toxicity from prior anti-cancer therapy that is > grade 1 and/or that is increasing in severity • Other severe or uncontrolled systemic disease or evidence of any other significant clinical disorder or lab findings that makes it undesirable for the patient to participate in the study • Any psychological, familial, sociological or geographical consideration potentially hampering compliance with study protocol • Known HIV or other chronic immunosuppressive disease • QTc that is immeasurable or > 480 msec on screening ECG

PLUTO – Study Eligibility Exclusion Criteria continued: • History of symptomatic peripheral vascular disease within 6 months prior to trial entry • Uncontrolled hypertension (BP > 150/90 at screening visit) • Evidence of active bleeding or bleeding diathesis • Recent haemoptysis • Patients who are unable or unwilling to withdraw potent CYP 3 A 4 inhibitors, inhibitors of P-glycoprotein or BCRP • Prior hypersensitivity to cremophor or known sensitivity to any component of pazopanib • Prior treatment with pazopanib or paclitaxel

PLUTO – Site Set Up CTU GLASGOW Main REC approval - MHRA approval - Site Initiation Slides - Investigator File - Pharmacy File SITE Staff Contact & Responsibilities Sheets – SSI - R&D Approval - Site Staff CVs and GCP certificates - Clinical Trial Agreement - - PIS, Consent, GP Letter etc on Trust Headed paper - Lab normal ranges (Haem + Biochem), Accreditation certificates. INITIATION PROCESS DRUG SUPPLY OF PAZOPANIB SITE ACTIVATED

PLUTO – Informed Consent Process • Two original Consent Forms must be completed by a clinician (or deputy listed on Staff Contacts & Responsibilities Sheet) • Two originals signed and completed by the patient • Date must be prior to registration/ randomisation. • Make one photocopy - Original to be filed in Investigator File - Original to be given to patient (+PIS) - Photocopy to be filed in hospital notes • Consent Form must not be sent to your coordinating trials office • Consent Notification Form must be completed and sent CR-UK Clinical Trials Unit, Glasgow • Errors noticed after consent - Add explanatory note/file note • New version of Patient Information Sheet must be provided to patients consented with previous version - Give to all patients regardless of treatment stage, during clinic visit • Patients who are still on active treatment will be required to repeat the consent process using the updated form - If not appropriate to re-consent patient (i. e. patient terminally ill) please make a note regarding this in the patients case notes and on re- consent log which is filed in your study site file.

PLUTO – Consent Withdrawal • This is when the patient specifically asks to withdraw their consent at any point in the study • Ensure that the level of consent withdrawal is clearly documented in the source data • If this occurs: – Document clearly in the patient notes that the patient has withdrawn consent, the level of consent withdrawal and the reason (if the patient has given any); – Complete the consent withdrawal notification form – Send the consent withdrawal notification form to the CR-UK CTU – No further follow-up should be collected on the patient from that point onwards.

PLUTO – Randomisation Process • All patients must be randomised onto the study prior to commencement of any treatment. • Check that patient has given written informed consent as per the informed consent process. • Check that patient fulfils eligibility criteria as per study protocol. • Complete Registration Form. • Site staff must contact the Cancer Research Clinical Trials Unit, Glasgow to randomise the patient. Registration to the study can be done by either telephone or fax on the following numbers: Tel no: ++ 44 141 301 7232 Fax no: ++ 44 141 301 7228* 08. 30 -17. 00 Mon-Thurs 08. 30 -16. 30 Friday, except public holidays * Faxes received outside of office hours will be processed the next working day • Each patient randomised will be allocated a unique sequential patient ID number for the study.

PLUTO – Pre Randomisation Evaluations Prior to commencing any study related procedures, all participants will be fully informed about the risks, benefits and procedures involved in study participation, and will sign a consent form confirming this process. All patients will then undergo a period of screening during the 28 days prior to initiation of study treatment. Screening will consist of: • • Complete medical history including diagnosis of bladder cancer and comorbidities Concomitant medication and their indications Physical examination including weight, height and performance status CT or MRI of chest pelvis and abdomen reported to RECIST v 1. 1

PLUTO - Pre randomisation Evaluations In addition, during the 7 days prior to initiation of study treatment all patients will undergo the following evaluations: • • • Baseline symptoms evaluation Vital signs including pulse and BP ECG (if QTC > 480 msec repeat until average result obtained) Haematology including FBC and Coag Biochemistry Pregnancy test Archived paraffin embedded tissue collection* Plasma blood sample collection* Whole blood sample collection* Urine Sample collection* Protocol treatment must commence within 7 days of registration. * Only applicable for patients participating in the translational component of the trial.

PLUTO -Treatment and Duration Arm A: • Patients will receive paclitaxel for up to 24 weeks. Infusions of paclitaxel 80 mg/m 2 will be administered on day 1, day 8 and day 15 of each 28 day cycle Arm B: • Patients will receive pazopanib 800 mg po od until progression (or toxicity/patient choice)

PLUTO –Pazopanib Dose Modifications Patients will be monitored closely for toxicity and the pazopanib dose may be adjusted according to individual patient tolerance If dose reduction is necessary, two dose reductions are permitted in stepwise fashion (initially to 600 mg and subsequently 400 mg). A direct dose reduction from 800 mg to 400 mg is also permitted in high toxicity. If the toxicity does not recur or worsen, the dose can then be increased step wise back to 600 mg and 800 mg after monitoring for 10 -14 days at each step if toxicity does not recur or worsen Dose interruptions are permitted for up to 14 days after which the patient should come off study. Participating centres are encouraged to liaise with the PLUTO trials office if the delay is over 7 days.

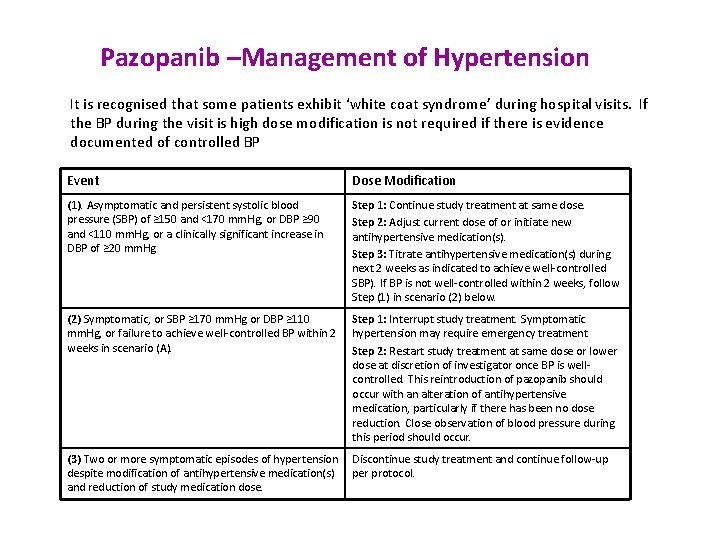
Pazopanib –Management of Hypertension It is recognised that some patients exhibit ‘white coat syndrome’ during hospital visits. If the BP during the visit is high dose modification is not required if there is evidence documented of controlled BP Event Dose Modification (1). Asymptomatic and persistent systolic blood pressure (SBP) of ≥ 150 and <170 mm. Hg, or DBP ≥ 90 and <110 mm. Hg, or a clinically significant increase in DBP of ≥ 20 mm. Hg Step 1: Continue study treatment at same dose. Step 2: Adjust current dose of or initiate new antihypertensive medication(s). Step 3: Titrate antihypertensive medication(s) during next 2 weeks as indicated to achieve well-controlled SBP). If BP is not well-controlled within 2 weeks, follow Step (1) in scenario (2) below. (2) Symptomatic, or SBP ≥ 170 mm. Hg or DBP ≥ 110 mm. Hg, or failure to achieve well-controlled BP within 2 weeks in scenario (A). Step 1: Interrupt study treatment. Symptomatic hypertension may require emergency treatment Step 2: Restart study treatment at same dose or lower dose at discretion of investigator once BP is wellcontrolled. This reintroduction of pazopanib should occur with an alteration of antihypertensive medication, particularly if there has been no dose reduction. Close observation of blood pressure during this period should occur. (3) Two or more symptomatic episodes of hypertension Discontinue study treatment and continue follow-up despite modification of antihypertensive medication(s) per protocol. and reduction of study medication dose.

Pazopanib - Management of Treatment Emergent Hepatotoxicity Event Dose Modification (A). ALT of ≤ 3. 0 x ULN Continue pazopanib at current dose with full panel LFTs monitored as per protocol (B) ALT > 3. 0 ULN to ≤ 8. 0 ULN without bilirubin elevation (defined as total bilirubin < 1. 5 x ULN or direct bilirubin ≤ 35%) and without hypersensitivity symptoms (e. g. fever, rash) Step 1: Continue pazopanib at current dose levels Step 2: Monitor subject closely for clinical signs and symptoms; perform full panel LFTs weekly or more frequently if clinically indicated until AST/ALT is reduced to grade 1 (C) ALT > 8. 0 x ULN without bilirubin elevation (defined as total bilirubin < 1. 5 x ULN or direct bilirubin ≤ 35%) and without hypersensitivity symptoms (e. g. fever, rash) 1 st Occurrence: Step 1: Interrupt pazopanib until toxicity resolves to ≤ grade 1 or baseline. Report the event to CTU Pharmacovigilance as an SAE within 24 hours of learning of its occurrence. Make every reasonable attempt to have subjects return to the clinic within 24 to 72 hours for repeat liver chemistries and liver event follow-up assessments Step 2: Liver imaging and other laboratory investigations should be considered as clinically appropriate Step 3: Monitor subjects closely for clinical signs and symptoms; perform full panel LFTs weekly or more frequently if clinically indicated until AST/ALT is reduced to grade 1 Step 4: If the subject is benefitting from study treatment , contact Chief Investigator for possible re-challenge. Re-treatment may be considered if ALL following criteria are met: -AST/ALT reduced to grade 1 -Total bilirubin < 1. 5 x ULN or direct bilirubin < 35% -No hypersensitivity signs or symptoms -Subject is benefitting from therapy Recurrence Discontinue pazopanib permanently and monitor subjects closely for clinical signs and symptoms; perform full panel LFTs weekly or more frequently if clinically indicated until ALT/AST is reduced to grade 1

Pazopanib – Management of prolonged QTc If QTc > 500 msec or an increase of > 60 msec from baseline is noted on a scheduled or unscheduled ECG then 2 additional ECGs should be obtained over a brief period of time (e. g. within 15 -20 mins) to confirm abnormality. The average QTc will be determined from the 3 readings and will be used to determine the appropriate next steps. If the average QTc ≤ 500 msec and < 60 msec increased from baseline then the subject can continue therapy If the average QTc is > 500 msec or > 60 msec increased from baseline the following steps should be taken • Study treatment should be interrupted immediately • Electrolytes, particularly k and mg should be checked and corrected if abnormal • Con meds with a potential for QTc interval prolongation should be discontinued • A cardiologist should be consulted to assist with the management of the subject • Communicate findings to PLUTO trials office

Pazopanib –Dose Reduction for other toxicities Haematological Platelets – No dose modification or interruption is required is the platelet count remains ≥ 50 x 109 l Neutrophils – No dose modification or interruption is required if the neutrophil count remains ≥ 1. 0 x 109/l Other Adverse Events Grade 1 or 2 – continue study treatment at same dose; monitor and treat as clinically indicated. Grade 3/4 Step 1 – interrupt study treatment until toxicity reduces to ≤ grade 1 Step 2 – Restart study treatment at same dose or lower at discretion of investigator Recurrent grade 3/4 Step 1 – interrupt study treatment until toxicity reduces to ≤ grade 1 Step 2 – Restart study treatment at lower dose

Pazopanib –Prohibited Concomitant Therapies Co-administration of pazopanib and medications which are substrates for the CYP 450 enzymes and have the potential to cause serious or life-threatening adverse events is prohibited from 14 days prior to the first dose of pazopanib through to study discontinuation. These medications include: Strong inhibitors of CYP 3 A 4 (see appendix VI) Some oral hypoglycemics Ergot derivatives Some neuroleptics Some antiarrhythmics Some immune modulators Miscellaneous (theophylline, quetiapine, risperidone, clozapine, )

Pazopanib Compliance • Empty pazopanib bottles and any remaining medication is to be returned at each patient visit. • These are then returned to pharmacy where a count takes place. • Sites will need to have a local process where pharmacy and research staff review patient returns in a timely manner in order to assess patient compliance. • Over or under compliance should be escalated to the study team. • Any patient non-compliant for more than 14 days should be taken off study.

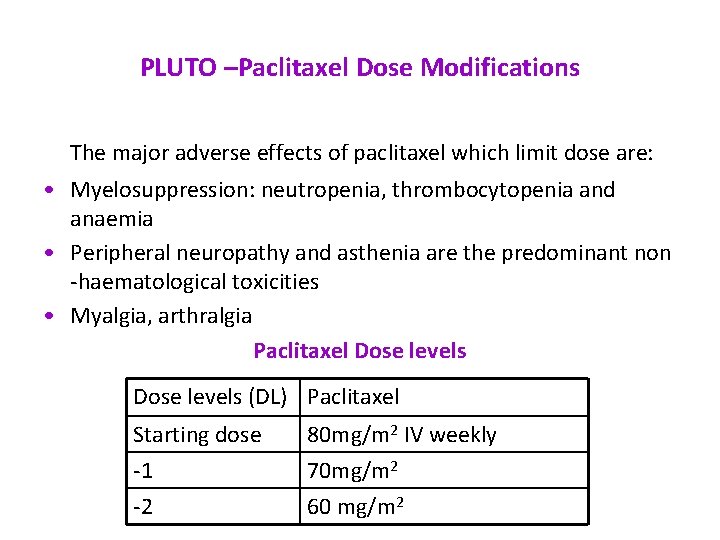
PLUTO –Paclitaxel Dose Modifications The major adverse effects of paclitaxel which limit dose are: • Myelosuppression: neutropenia, thrombocytopenia and anaemia • Peripheral neuropathy and asthenia are the predominant non -haematological toxicities • Myalgia, arthralgia Paclitaxel Dose levels (DL) Paclitaxel Starting dose -1 -2 80 mg/m 2 IV weekly 70 mg/m 2 60 mg/m 2

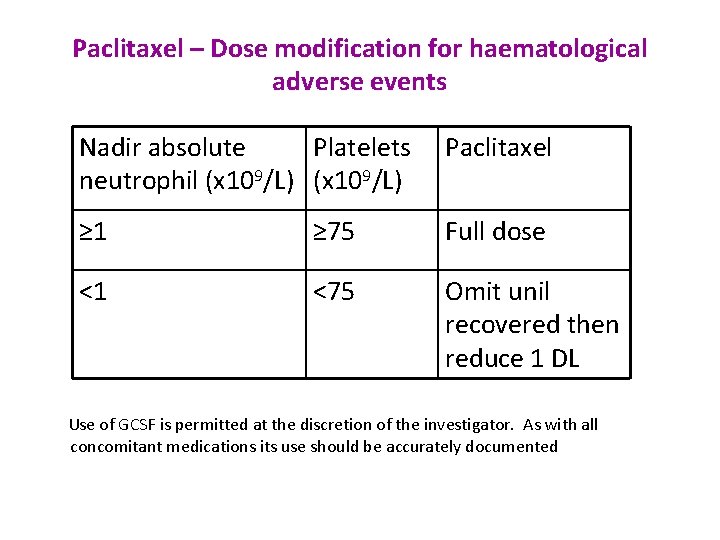
Paclitaxel – Dose modification for haematological adverse events Nadir absolute Platelets Paclitaxel neutrophil (x 109/L) ≥ 1 ≥ 75 Full dose <1 <75 Omit unil recovered then reduce 1 DL Use of GCSF is permitted at the discretion of the investigator. As with all concomitant medications its use should be accurately documented

Paclitaxel – Dose modification for non-haematological toxicity Event Management / Next Paclitaxel dose ≤ grade 1 No change in dose Grade 2 Omit paclitaxel until ≤ grade 1 – resume at same dose * (*excludes alopecia. Patients with grade 2 neurotoxicity will require a dose reduction Grade 3 or 4** / ***** Hold*** until ≤ grade 1 – resume at 1 DL if indicated**** (**excludes brief, sub-optimally managed (***patients requiring more than to treatment omissions nausea and vomiting, rash, arthralgia and should go off protocol therapy) myalgia) (*****In the case of grade 3 or 4 cardiac failure study treatment will be discontinued) (****patients requiring more than 2 dose reductions should go off protocol therapy)

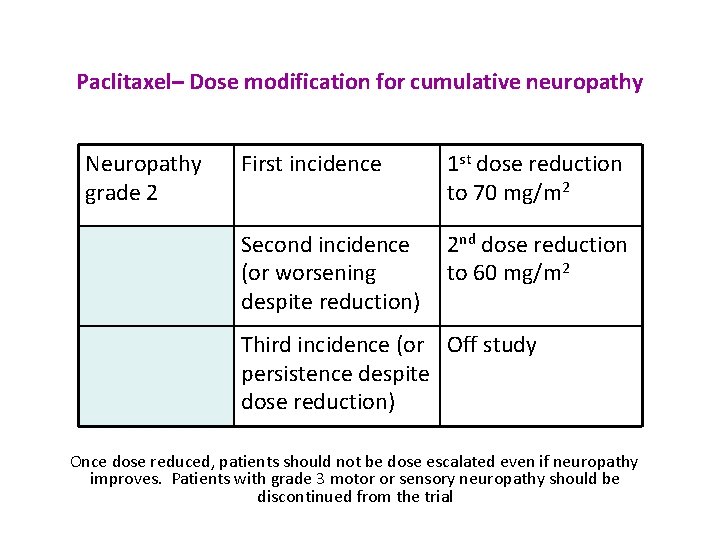
Paclitaxel– Dose modification for cumulative neuropathy Neuropathy grade 2 First incidence 1 st dose reduction to 70 mg/m 2 Second incidence (or worsening despite reduction) 2 nd dose reduction to 60 mg/m 2 Third incidence (or Off study persistence despite dose reduction) Once dose reduced, patients should not be dose escalated even if neuropathy improves. Patients with grade 3 motor or sensory neuropathy should be discontinued from the trial

Paclitaxel –Concomitant Therapies Caution should be exercised during concurrent administration of active substances which are metabolised in the liver as these may inhibit the metabolism of paclitaxel. Caution should be exercised when administering paclitaxel concomitantly with medicines known to inhibit or induce either CYP 2 C. Strong inhibitors of CYP 3 A 4 are prohibited during administration of Pazopanib

PLUTO - Translational Research • The most recent archived paraffin embedded tissue available will be collected and stored for future biomarker studies • Whole blood samples will be collected at baseline for pharmacogenomics • Plasma and urine samples will be taken at baseline, weeks 4 and 12 and at confirmed progression • The blood and urine sample collection aspect of the trial is optional to patients, and samples should only be collected for patients who have consented to this. • The samples must be collected for all patients who have consented to the translational aspects of the study on the consent form, please ensure all samples are taken for all consenting patients

PLUTO - Assessment and Follow Up Formal clinical assessment for week 1 -25: • During the first 24 weeks of the study patients should be seen formally on a 4 weekly basis in clinic. • Radiological assessments will occur at 12 weekly (+/- 7 days) intervals until disease progression - CT or MRI scan of chest, abdomen and pelvis reported to RECIST v 1. 1 • ECG - only required for patients receiving pazopanib (Arm B) • ECOG Performance Status • Toxicity Assessment • Concomitant medication review • Blood pressure and pulse • Haematology - including full blood count with WBC, ANC, platelets count and haemoglobin • Biochemistry - including LFTs (including ALT, bilirubin), U + E, magnesium, calcium, alkaline phosphatase, potassium, LDH, C-reactive protein and albumin. • Urinalysis • Plasma Blood sample collection** • Urine sample collection** **Only applicable to centres/patients participating in the translational component of the trial.

PLUTO - Assessment and Follow Up From 24 weeks onwards all patients will be seen on a 6 weekly basis 6 -weekly from Week 31 onwards until progression or death • Both Arm A and Arm B patients should receive: • ECOG Performance Status • Toxicity Assessment (if pazopanib ongoing or if unresolved toxicity) • Arm B (pazopanib) patients should also receive: • Concomitant medication review (if pazopanib ongoing) • Blood pressure and pulse • Haematology - including full blood count with WBC, ANC, platelets count and haemoglobin • Biochemistry - including LFTs (including ALT, bilirubin), U + E, magnesium, calcium, alkaline phosphatase, potassium, LDH, C-reactive protein and albumin • Urinalysis • Thyroid function testing (12 weekly if pazopanib ongoing) 12 -weekly from Week 31 onwards Both Arm A and Arm B patients should receive: • Radiological Assessment – CT scan of the chest, abdomen and pelvis, or MRI. The same modality should be used as for the baseline/previous 12 weekly scans. Scans should be performed 12 weekly until progression. A window of +/- 7 days is permitted. Scans must be reported to RECIST v 1. 1 • FACT-Bl (Qo. L) form (to be collected 12 weekly from Week 37 onwards) Assessments after 2 years Post Randomisation • For those patients still alive and progression-free after 24 months on trial, further follow up and cross sectional imaging will be at the discretion of the treating physician

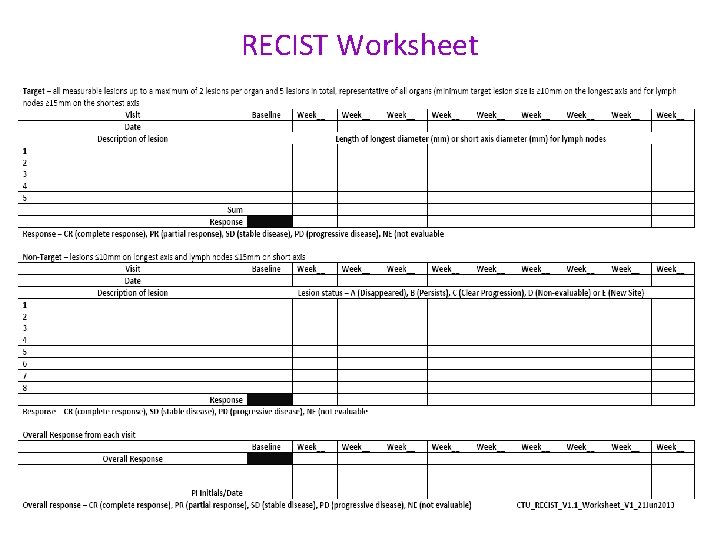
Reporting to RECIST • All radiological investigations must be reported as per protocol / RECIST version 1. 1. • Source documentation of this must be available for review if the original report has had to be supplemented to bring it in line with protocol requirements. • CTU, Glasgow have produced a worksheet to assist with the documentation of study specific reporting and will make this available to any participating site upon request to the study monitor.

RECIST Worksheet

PLUTO - CRFs • • • Consent Notification Form Randomisation Form Pre-treatment Form FACT-Bl Qo. L Form Treatment Form Response Form Follow–up Form Concomitant Medication Form Consent Withdrawal Notification Form Pregnancy Notification Form

PLUTO – CRF Completion • CRF completion guidelines for the study are currently being developed and will provided to sites when available • Entries to the CRFs will be made in black ball-point pen and must be legible • Correction fluid etc. must not be used • Any errors must be crossed out with a single stroke, correction inserted and change initialled and dated • An explanation can be written next to amendment if necessary • Date format: DD/ MON/ YYYY • Please ensure all data submitted on the CRFs is verifiable in source documents • Take photocopy of all completed CRFs. Originals to be sent to CR-UK CTU Glasgow

PLUTO – CRF Completion Timelines for entry of required data in the CRF Timelines for resolution of data queries Timelines for receipt of CRFs at CR-UK CTU, Glasgow Within 4 weeks of patient visit Within 4 weeks of receipt Within 2 weeks of form completion

PLUTO Trial - Pharmacovigilance Clinical Trial Regulations require: • Investigators document Adverse Events (AEs) in patient notes and the CRF • Investigators report Serious Adverse Events (SAEs) immediately to the CR-UK Clinical Trials Unit, Glasgow (CTU) • The CTU (on behalf of the Sponsor) make expedited reports of Suspected Unexpected Serious Adverse Reactions (SUSARs) to the Regulatory Authority (MHRA), Main REC GSK & Sponsor • The CTU will produce the Development Safety Update Reports (DSURs)

PLUTO Trial – Adverse Event Reporting • All AEs must be followed: - until resolution, - or for at least 30 days after discontinuation of study medication, - or until toxicity has resolved to baseline, - or < Grade 1, - or until toxicity is considered to be irreversible • All AE and toxicities must be graded according to the NCI-CTCAE Version 4. 0 • An exacerbation of pre-existing condition is an AE

Definition of a SERIOUS ADVERSE EVENT A Serious Adverse Event (SAE) is defined as any untoward medical occurrence, not necessarily related to protocol treatment, that: • Results in death • Is life-threatening • Requires hospitalisation or prolongation of existing hospitalisation • Results in persistent or significant disability or incapacity • Consists of a congenital anomaly or birth defect • Is considered medically significant by the Investigator

Definition of a SERIOUS ADVERSE EVENT Life threatening: • The patient is at immediate risk of death from the event as it occurred. It does not include an event that, had it occurred in a more serious form, might have caused death Requires in-patient hospitalisation: • Is as a hospital admission required for treatment of an adverse event even when the adverse event is not related to the protocol treatment

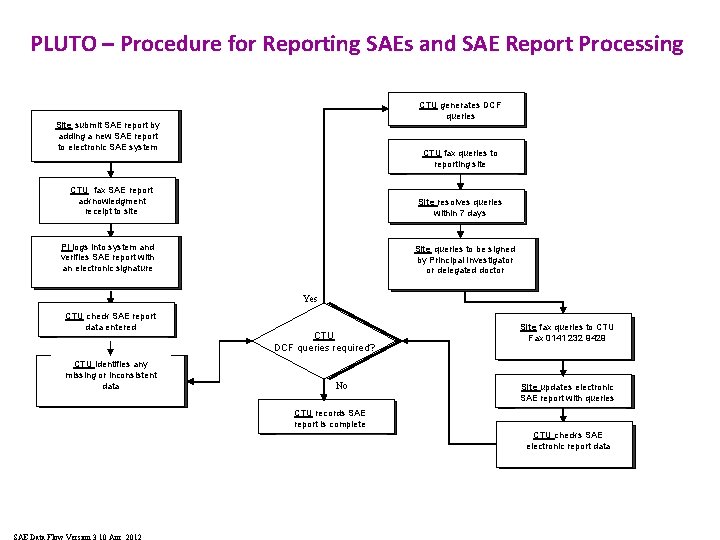
PLUTO - Reporting SAEs • SAEs must be reported immediately (within 24 hours of knowledge of the event) • SAEs are reported using an electronic system developed on behalf of the Sponsor by the Robertson Centre for Biostatistics • Sites input details for each SAE on the electronic system • The CTU will acknowledge receipt of each new initial report • The SAE report will be forwarded to GSK • The CTU will request additional information if the event is unexpected • CTU will raise queries for any inconsistent or missing information

PLUTO – Procedure for Reporting SAEs and SAE Report Processing CTU generates Patient SAE and DCF SAE queries form completed Site submit by Patient SAESAE and report SAE form adding a completed new SAE report to electronic SAE system CTU queries to Patientfax SAE and SAE reporting site form completed CTU fax. SAE report Patient and SAE acknowledgment form completed receipt to site Site resolves queries Patient SAE and SAE withincompleted 7 days form PI logs into system and Patient SAE and SAE form verifies SAE report with completed an electronic signature Site queries to be signed Patient SAE and SAE form by Principal Investigator completed or delegated doctor Yes CTU check SAE Patient SAE andreport SAE data completed entered form CTU identifies any Patient SAE and SAE form missing or inconsistent completed data Is SAE CTUrelated to IMA? DCF queries required? No CTU records Patient SAE and. SAE report complete formiscompleted SAE Data Flow Version 3 10 Apr 2012 Site fax queries to. SAE CTU Patient SAE and Faxform 0141 232 9429 completed Site updates Patient SAEelectronic and SAE report with queries form completed CTU checks SAE Patient SAE and SAE electronic report data form completed

PLUTO - Procedure for Identifying Unexpected and Related Events • A checklist will be used to identify SUSARs that require expedited reporting to the Regulatory Authority, Main REC and Sponsor • The checklist is a list of the events expected to occur in patients receiving the protocol treatment (Paclitaxel and Pazopanib). For any SAE that is documented as related to protocol treatment (SAR) and is not listed on the checklist, the Chief Investigator will be contacted for an opinion of SUSAR status (unexpectedness) • The Chief Investigator is responsible for deciding if a SAR requires expedited reporting • GSK will be informed of the decision to report a SAR as a SUSAR and receive a copy of the SUSAR report

PLUTO - Expedited Reporting SAEs that meet the criteria for SUSARs will be reported to the MHRA, Main REC, GSK and Sponsor where in the opinion of the Chief Investigator the event was: • Related – that is, resulted from administration of any of the research procedures And • Unexpected – that is the type of event is not listed in the Investigator Brochure or Summary of Product Characteristics (Sm. PC) as an expected occurrence Reports of related and unexpected SAEs will be submitted within 7 days for fatal/life threatening events and 15 days for all other events

PLUTO - Development Safety Update Reports A Development Safety Update Report (DSUR) covering the safety of all trial participants will be prepared by the CR-UK CTU and submitted to the MHRA, Main REC, GSK, Sponsor & trial Investigators annually

MONITORING (1) Central Monitoring Study sites will be monitored centrally by checking incoming forms for compliance with the protocol, data consistency, missing data and timing. Study staff will be in regular contact with site personnel (by phone/fax/email/letter) to check on progress and deal with any queries that they may have. On-site and Remote Telephone Monitoring The 1 st visit will take the form of a remote telephone monitoring visit: § The time & date will be agreed with a member of the Site Study Team & a separate time & date agreed with a member of the Clinical Trials Pharmacy Department § A pro forma covering the questions which will be covered during the telephone monitoring visit will be sent with confirmation of the agreed date § Please set aside 50 to 70 minutes for this call.

MONITORING (2) The 2 nd visit will take the form of an on site monitoring visit: • • Investigators and site staff will be notified in advance about forthcoming pre arranged monitoring visits All patient source documentation should be made available to enable Source Document Verification by the Clinical Trial Monitor • A full working day is required for on-site visits & arrangements should be in place to facilitate the monitor access on the agreed date • If sites are able to provide printed results/reports these must be filed in the source documents • If a site is using electronic data reporting systems or electronic records & hard copies are not available the clinical trial monitor must be permitted access to the system either by being issued with a temporary login or a member of staff available for the duration of the visit to facilitate electronic access to authorised reports/results • The pharmacy department responsible for the trial will be visited to allow monitoring of the pharmacy site file and review of security, storage and accountability of trial drugs. • All findings will be discussed at an end of visit and any unresolved issues raised as Action Points • Action Points will be followed up by the monitor until resolved

PLUTO – Ethical and Regulatory Standards • Study will be conducted according to ICH GCP guidelines • Study conducted in accordance with the EU Directive 2001/20/EC • Trial carried out in accordance with the World Medical Association Declaration of Helsinki (1964) and the Tokyo (1975), Venice (1983), Hong Kong (1989), South Africa (1996), Edinburgh (2000), Washington (2002), Tokyo (2004), Seoul (2008) amendments

PLUTO – Investigator Responsibilities The following principles are from ICH GCP Topic E 6 and apply to clinical trials of Investigational Medicinal Products: • • Qualifications & Agreements: The Investigator should be qualified by education, training & experience. Thoroughly familiar with protocol & medicinal products. Comply with GCP and applicable regulations. Permit – monitoring and audit by the sponsor and inspection by regulatory authorities. Maintain a delegation logs of staff involved in the clinical trial at the trial site. Ensure that all persons assisting with the trial are adequately informed about the protocol, IMP and their duties and functions. Resources: The Investigator should have sufficient time to properly conduct and complete the trial within the agreed period. Have available adequate facilities and qualified staff to conduct the trial properly and safely.

PLUTO – Investigator Responsibilities • • • Medical Care of Trial Subjects: - A qualified physician who is an Investigator (or co-investigator) should be responsible for all trial related medical decisions. During and following participation the Investigator should ensure adequate medical care for any adverse events (AEs). The Investigator should make as reasonable effort to ascertain reasons for withdrawal from the trial (although a subject is not obliged to give reasons) Ethics: Before initiating the trial there should be written and dated approval/favourable opinion from the Ethics Committee for the protocol, patient information sheet/consent form and any amendments. Compliance with Protocol: The Investigator should conduct the trial in compliance with the protocol. Not implement any deviation from the protocol without prior approval/favourable opinion of the IEC and the sponsor. The Investigator should document and explain any deviation from the protocol.

PLUTO – Investigator Responsibilities • The IMP : Investigator has responsibility for IMP accountability at trial site Some/all IMP duties at the trial site may be assigned to suitably qualified pharmacist. Records must be maintained: delivery, inventory, use and destruction - Storage of the IMP should be as specified by the sponsor/regulatory requirements. The IMP should only be used in accordance with the protocol. The Investigator (or designee) should explain the correct use of the IMP to each patient. • Randomisation: The Investigator should follow the trial’s randomisation procedures as detailed in the protocol. • Informed consent: In obtaining and documenting informed consent, the investigator should comply with the applicable regulatory requirement (s), and should adhere to GCP and to the ethical principles that have their origin in the Declaration of Helsinki.

PLUTO – Investigator Responsibilities • Reports & records – The investigator is responsible for accuracy, completeness, legibility and timeliness of the data reported to the sponsor. Data reported on CRFS, from source documents should be consistent with source documents or discrepancies explained. Corrections should be : dated, initialled, explained (if necessary) and should not obscure the original entry. All trial documents should be maintained as specified in ICH GCP E 6, Section 8 (Essential documents for the conduct of a clinical trial). • Safety reporting: Investigators must report Serious Adverse Events to the sponsor as soon as they become aware of the event.

PLUTO – Other Site Staff The Principal Investigator has overall responsibility for the conduct of the clinical trial at the trial site. BUT • • All staff must comply with GCP. Staff should only perform tasks delegated to them. Staff should ensure that their details are available to the Investigator. Staff should maintain appropriate confidentiality at all times

PLUTO - CONTACT DETAILS FOR CR-UK CTU Project Manager Judith Dixon Tel: 0141 301 7540 Fax: 0141 301 724 E-mail: judith. dixon@glasgow. ac. uk Trial Coordinator Anna Morris Tel: 0141 301 7232 Fax: 0141 301 7228 E-mail: anna. morris@glasgow. ac. uk Pharmacovigilance Manager Lindsey Connery Tel: 0141 301 7209 Fax: 0141 301 7213 E-mail: lindsey. connery@glasgow. ac. uk Pharmacovigilance CTC Susannah Radford Tel: 0141 301 7211 Fax: 0141 301 7213 E-mail: susannah. radford@glasgow. ac. uk CR-UK CTU, Glasgow Cancer Research UK Clinical Trials Office Level 0, Beatson West of Scotland Cancer Centre 1053 Great Western Road, Glasgow, G 12 0 YN