9 5 Temperaturedependence of reaction rate Arrhenius equation

- Slides: 24

§ 9. 5 Temperature-dependence of reaction rate -- Arrhenius equation It’s the time for you to compose your course thesis!

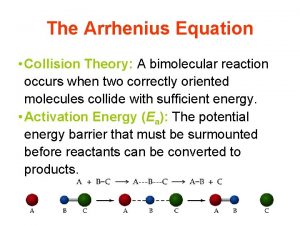
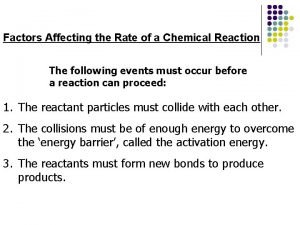
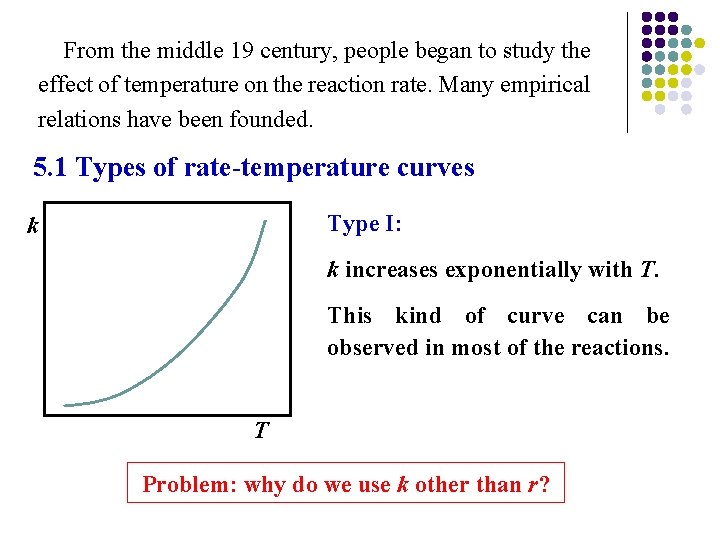
From the middle 19 century, people began to study the effect of temperature on the reaction rate. Many empirical relations have been founded. 5. 1 Types of rate-temperature curves Type I: k k increases exponentially with T. This kind of curve can be observed in most of the reactions. T Problem: why do we use k other than r?

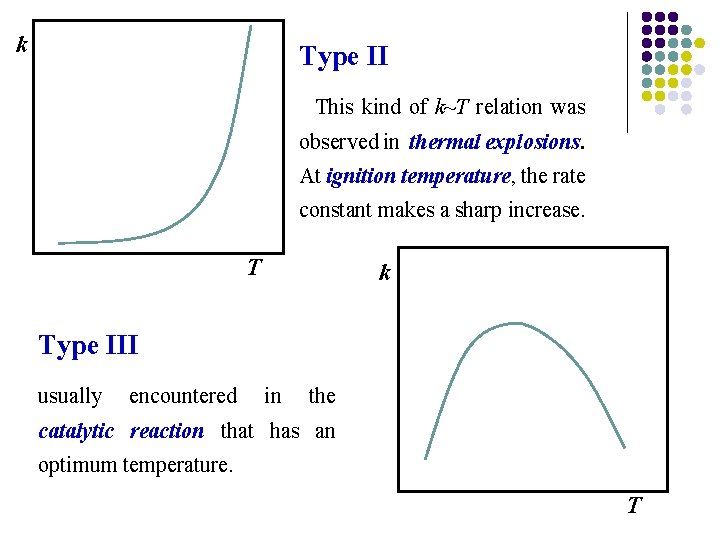
k Type II This kind of k~T relation was observed in thermal explosions. At ignition temperature, the rate constant makes a sharp increase. T k Type III usually encountered in the catalytic reaction that has an optimum temperature. T

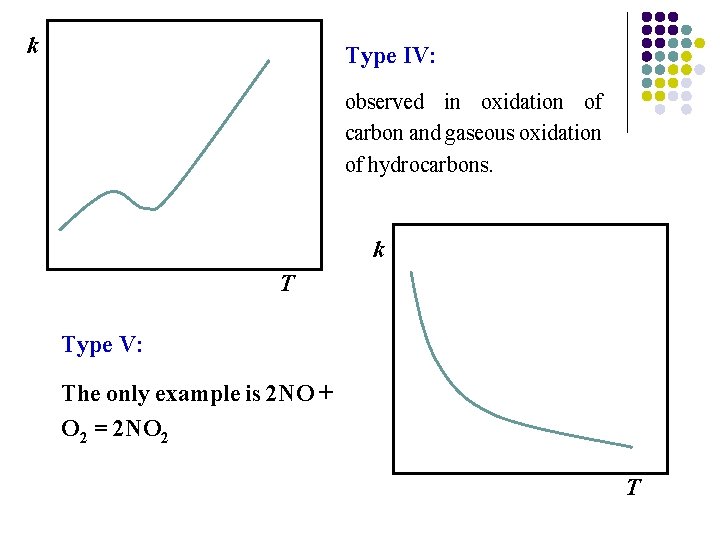
k Type IV: observed in oxidation of carbon and gaseous oxidation of hydrocarbons. k T Type V: The only example is 2 NO + O 2 = 2 NO 2 T

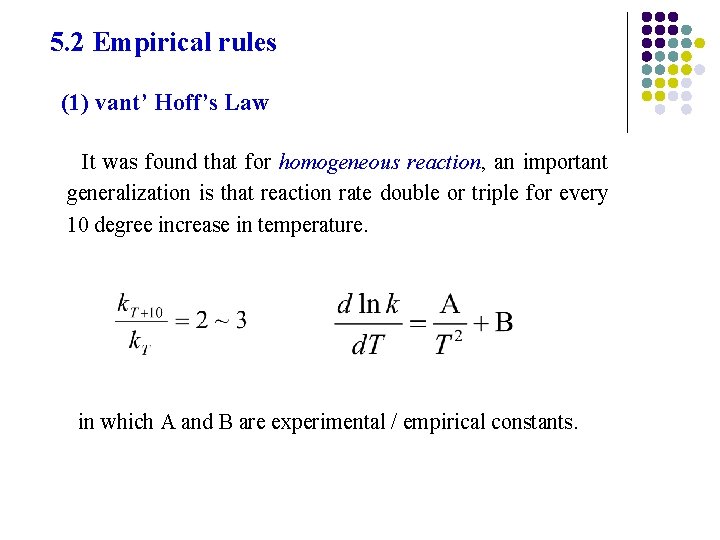
5. 2 Empirical rules (1) vant’ Hoff’s Law It was found that for homogeneous reaction, an important generalization is that reaction rate double or triple for every 10 degree increase in temperature. in which A and B are experimental / empirical constants.

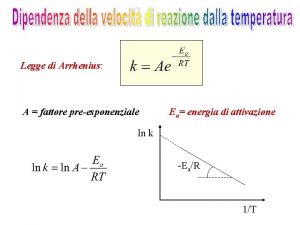
(2) Arrhenius equation In 1889, Arrhenius made detailed theoretical consideration on the hydrolysis of sucrose. C 12 H 22 O 11 + H 2 O C 6 H 12 O 6 + C 6 H 12 O 6 in which sucrose molecules were surrounded by water, if all sucrose molecules could react directly with water, the reaction should completed instantly. However, this is not the case. Arrhenius postulated that only a small part of sucrose molecules with higher energy (activated molecules) can react with water and, therefore, the reaction can only proceed at a low rate. By taking enough energy, the common sucrose molecules can change into activated molecules. The energy needed for this conversion was called activation energy. [A very important concept!]

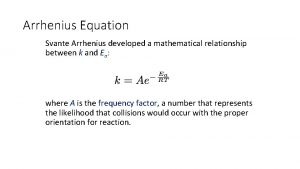
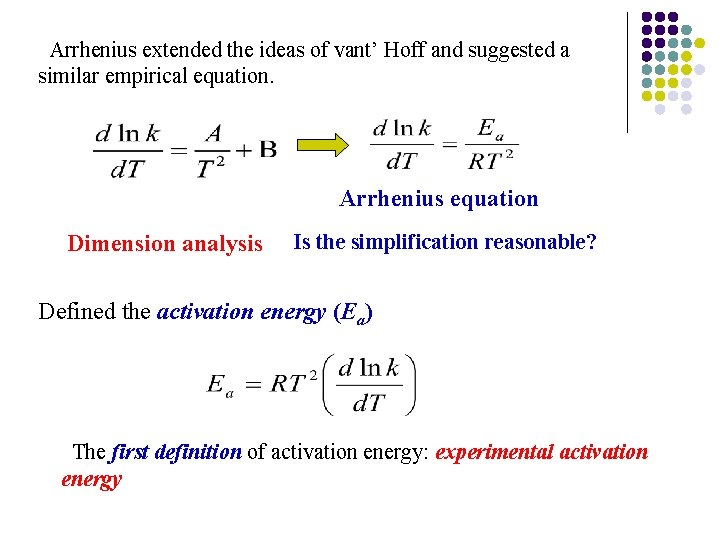
Arrhenius extended the ideas of vant’ Hoff and suggested a similar empirical equation. Arrhenius equation Dimension analysis Is the simplification reasonable? Defined the activation energy (Ea) The first definition of activation energy: experimental activation energy

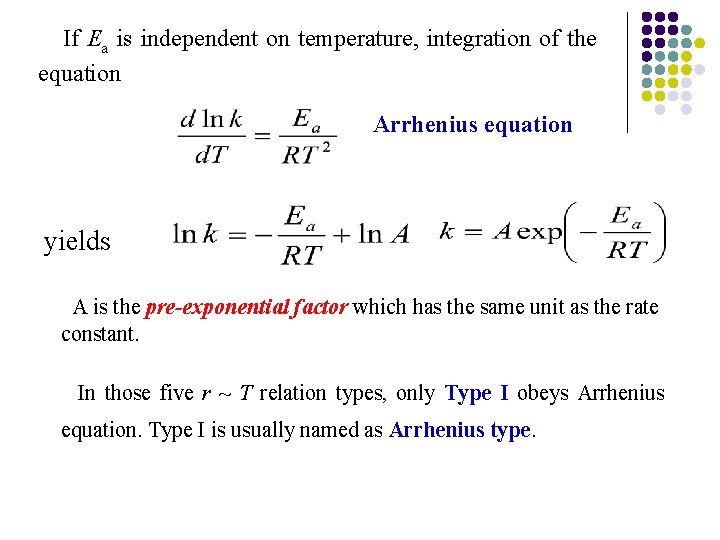
If Ea is independent on temperature, integration of the equation Arrhenius equation yields A is the pre-exponential factor which has the same unit as the rate constant. In those five r ~ T relation types, only Type I obeys Arrhenius equation. Type I is usually named as Arrhenius type.

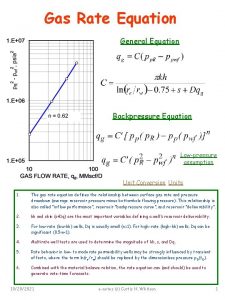
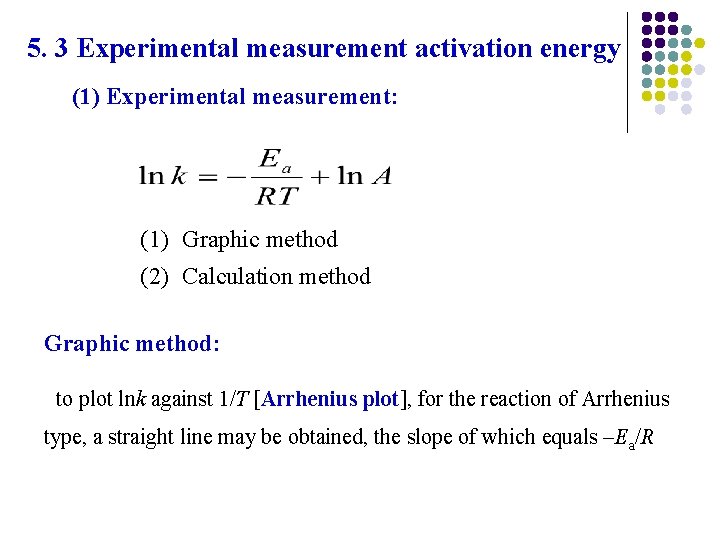
5. 3 Experimental measurement activation energy (1) Experimental measurement: (1) Graphic method (2) Calculation method Graphic method: to plot lnk against 1/T [Arrhenius plot], for the reaction of Arrhenius type, a straight line may be obtained, the slope of which equals –Ea/R

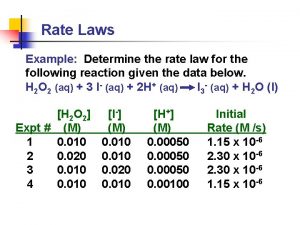
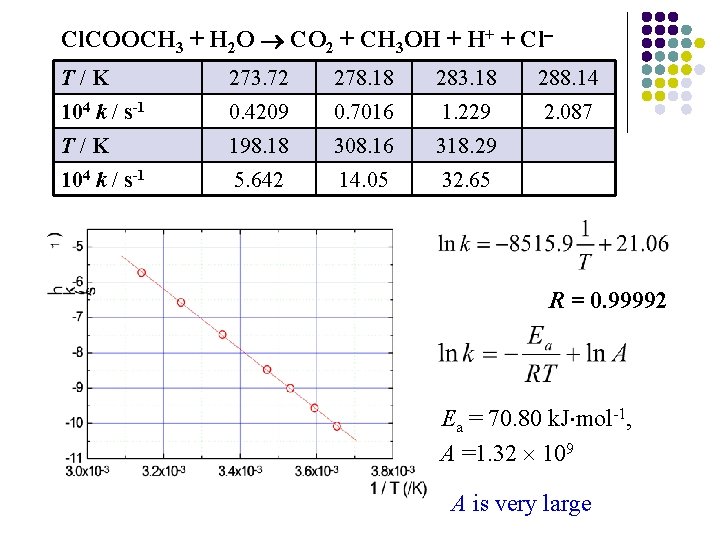
Cl. COOCH 3 + H 2 O CO 2 + CH 3 OH + H+ + Cl T/K 273. 72 278. 18 283. 18 288. 14 104 k / s-1 0. 4209 0. 7016 1. 229 2. 087 T/K 198. 18 308. 16 318. 29 104 k / s-1 5. 642 14. 05 32. 65 R = 0. 99992 Ea = 70. 80 k. J mol-1, A =1. 32 109 A is very large

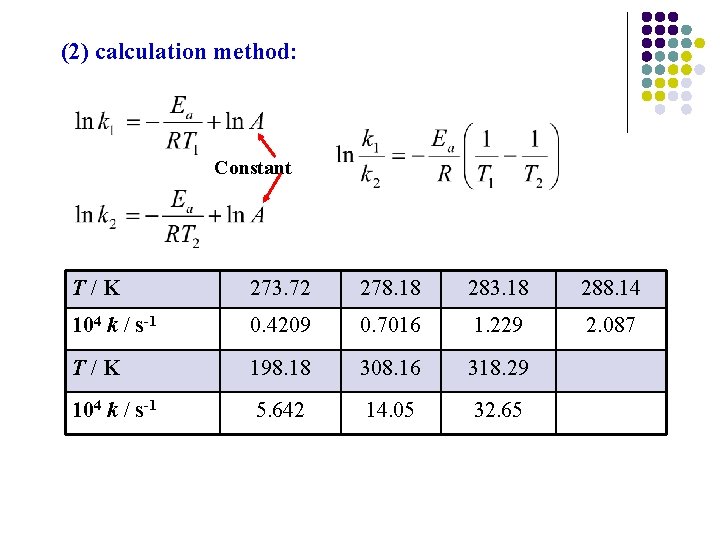
(2) calculation method: Constant T/K 273. 72 278. 18 283. 18 288. 14 104 k / s-1 0. 4209 0. 7016 1. 229 2. 087 T/K 198. 18 308. 16 318. 29 104 k / s-1 5. 642 14. 05 32. 65

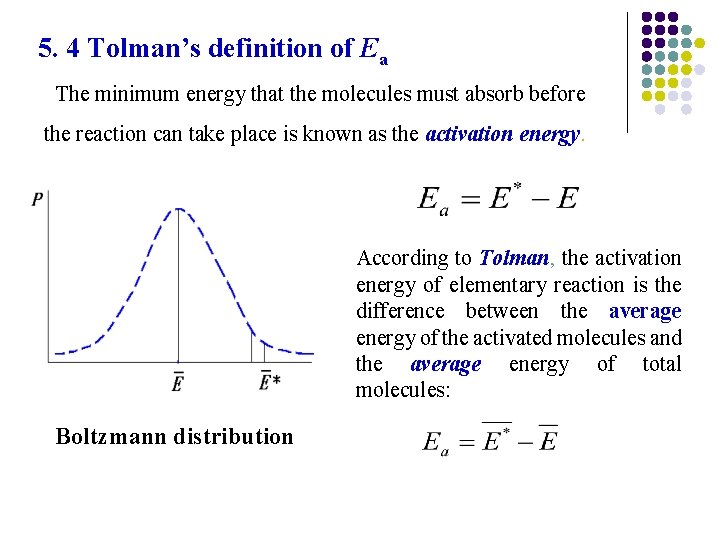
5. 4 Tolman’s definition of Ea The minimum energy that the molecules must absorb before the reaction can take place is known as the activation energy. According to Tolman, the activation energy of elementary reaction is the difference between the average energy of the activated molecules and the average energy of total molecules: Boltzmann distribution

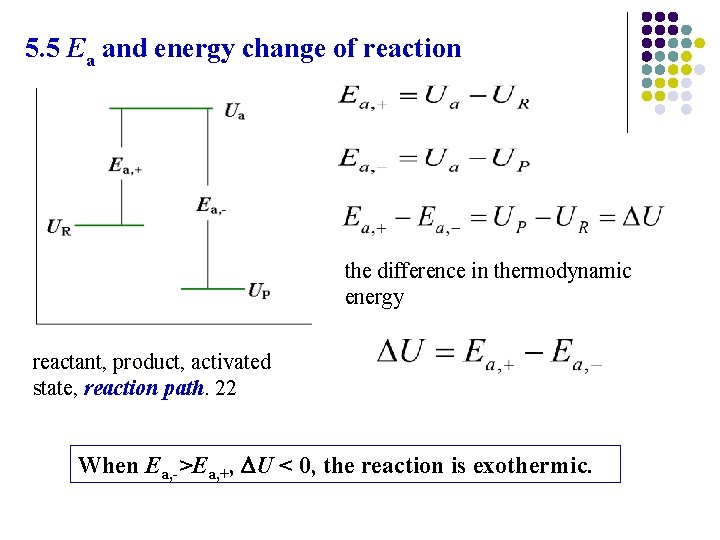
5. 5 Ea and energy change of reaction the difference in thermodynamic energy reactant, product, activated state, reaction path. 22 When Ea, ->Ea, +, U < 0, the reaction is exothermic.

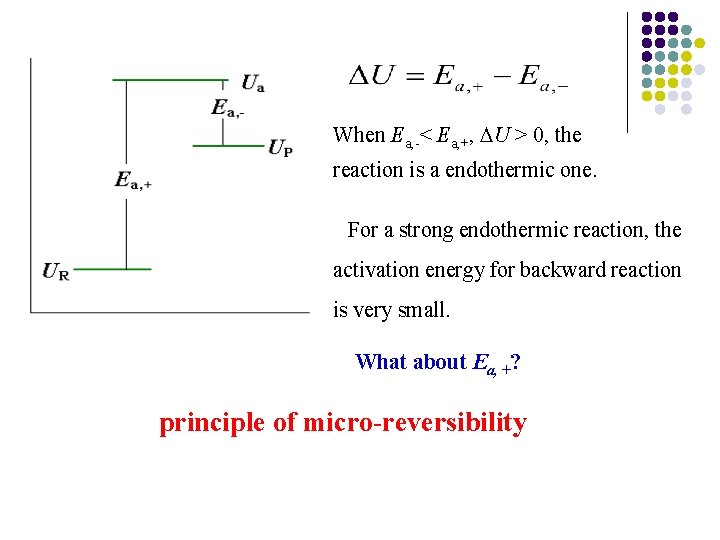
When Ea, -< Ea, +, U > 0, the reaction is a endothermic one. For a strong endothermic reaction, the activation energy for backward reaction is very small. What about Ea, +? principle of micro-reversibility

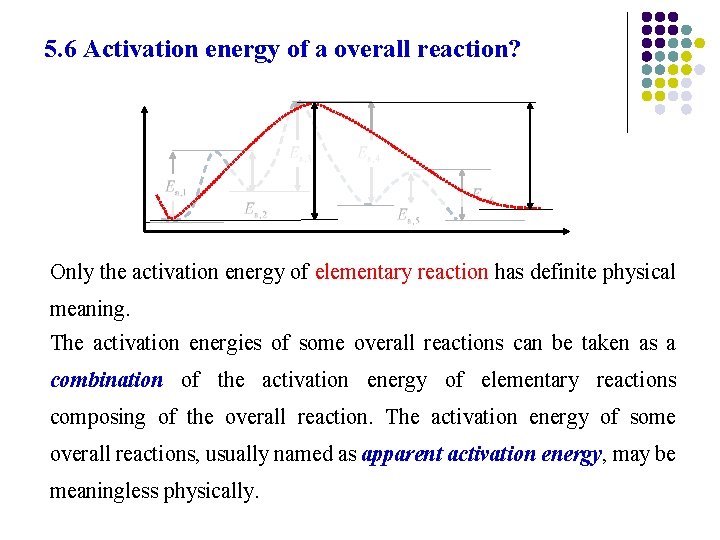
5. 6 Activation energy of a overall reaction? Only the activation energy of elementary reaction has definite physical meaning. The activation energies of some overall reactions can be taken as a combination of the activation energy of elementary reactions composing of the overall reaction. The activation energy of some overall reactions, usually named as apparent activation energy, may be meaningless physically.

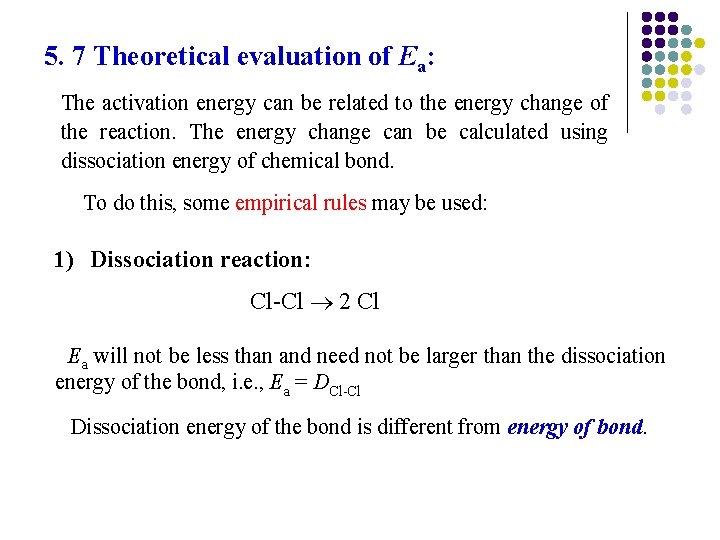
5. 7 Theoretical evaluation of Ea: The activation energy can be related to the energy change of the reaction. The energy change can be calculated using dissociation energy of chemical bond. To do this, some empirical rules may be used: 1) Dissociation reaction: Cl-Cl 2 Cl Ea will not be less than and need not be larger than the dissociation energy of the bond, i. e. , Ea = DCl-Cl Dissociation energy of the bond is different from energy of bond.

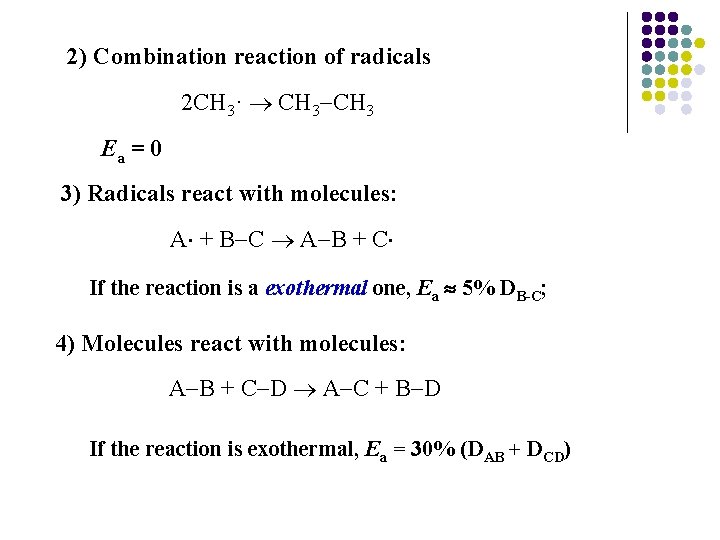
2) Combination reaction of radicals 2 CH 3· CH 3 Ea = 0 3) Radicals react with molecules: A + B C A B + C If the reaction is a exothermal one, Ea 5% DB-C; 4) Molecules react with molecules: A B + C D A C + B D If the reaction is exothermal, Ea = 30% (DAB + DCD)

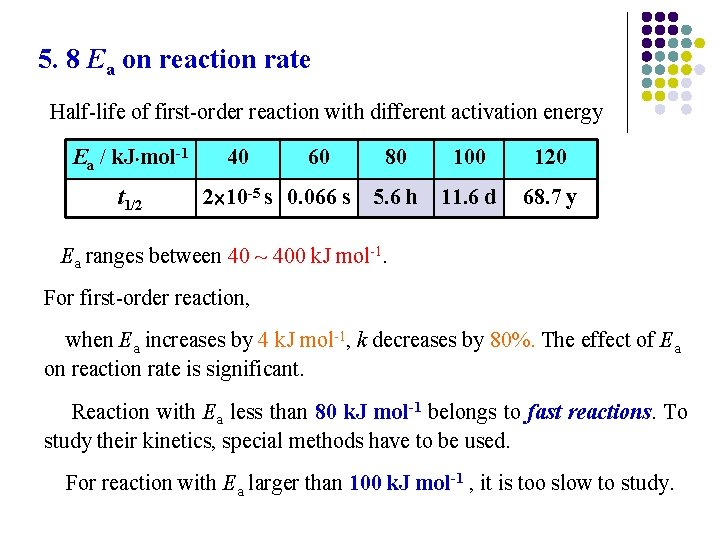
5. 8 Ea on reaction rate Half-life of first-order reaction with different activation energy Ea / k. J mol-1 t 1/2 40 60 2 10 -5 s 0. 066 s 80 100 120 5. 6 h 11. 6 d 68. 7 y Ea ranges between 40 ~ 400 k. J mol-1. For first-order reaction, when Ea increases by 4 k. J mol-1, k decreases by 80%. The effect of Ea on reaction rate is significant. Reaction with Ea less than 80 k. J mol-1 belongs to fast reactions. To study their kinetics, special methods have to be used. For reaction with Ea larger than 100 k. J mol-1 , it is too slow to study.

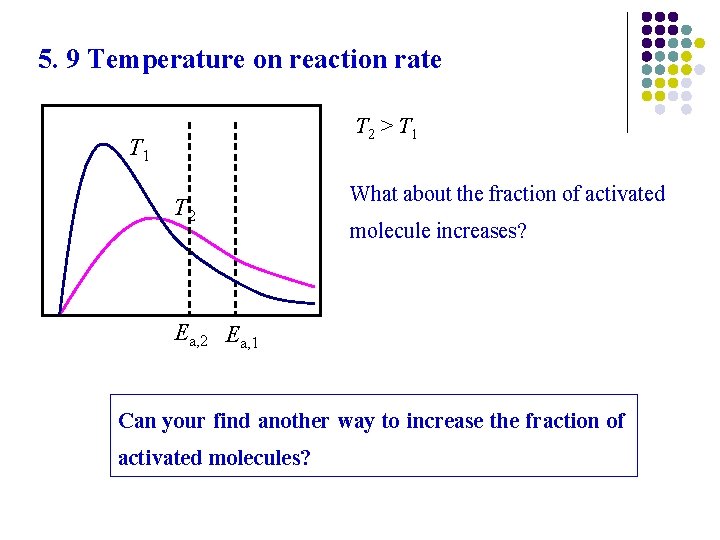
5. 9 Temperature on reaction rate T 2 > T 1 T 2 What about the fraction of activated molecule increases? Ea, 2 Ea, 1 Can your find another way to increase the fraction of activated molecules?

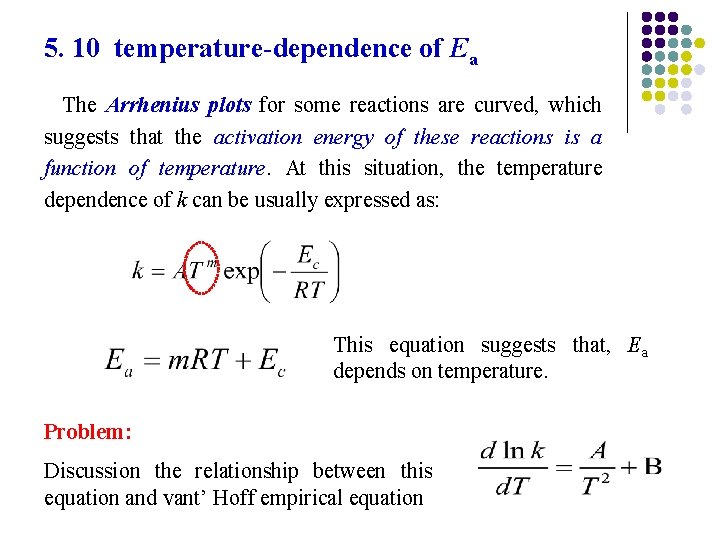
5. 10 temperature-dependence of Ea The Arrhenius plots for some reactions are curved, which suggests that the activation energy of these reactions is a function of temperature. At this situation, the temperature dependence of k can be usually expressed as: This equation suggests that, Ea depends on temperature. Problem: Discussion the relationship between this equation and vant’ Hoff empirical equation

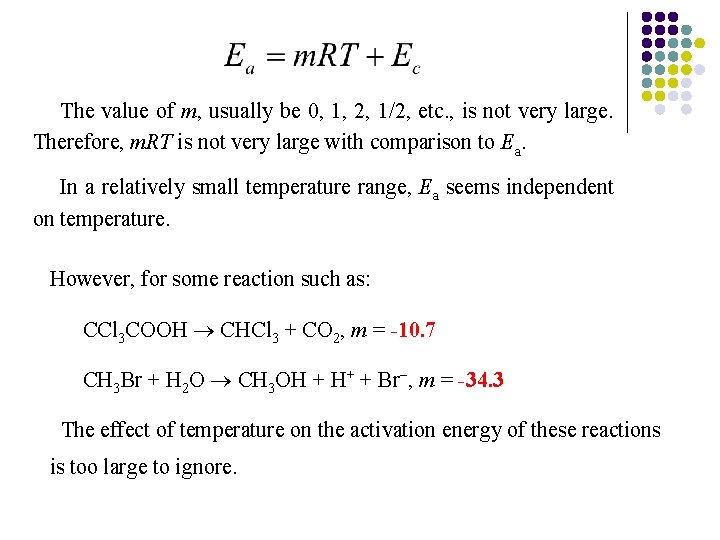
The value of m, usually be 0, 1, 2, 1/2, etc. , is not very large. Therefore, m. RT is not very large with comparison to Ea. In a relatively small temperature range, Ea seems independent on temperature. However, for some reaction such as: CCl 3 COOH CHCl 3 + CO 2, m = -10. 7 CH 3 Br + H 2 O CH 3 OH + H+ + Br , m = -34. 3 The effect of temperature on the activation energy of these reactions is too large to ignore.

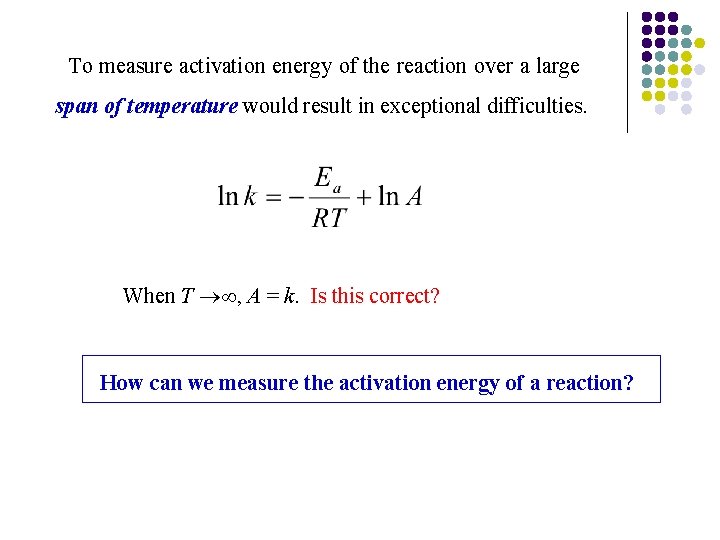
To measure activation energy of the reaction over a large span of temperature would result in exceptional difficulties. When T , A = k. Is this correct? How can we measure the activation energy of a reaction?

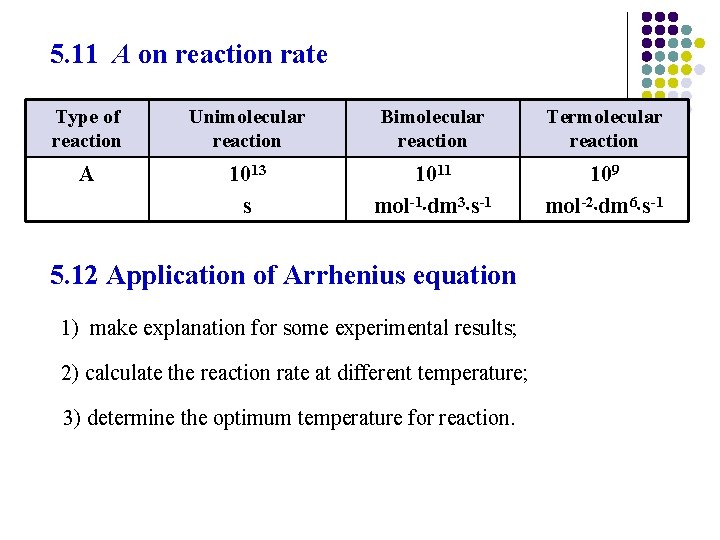
5. 11 A on reaction rate Type of reaction Unimolecular reaction Bimolecular reaction Termolecular reaction A 1013 s 1011 mol-1 dm 3 s-1 109 mol-2 dm 6 s-1 5. 12 Application of Arrhenius equation 1) make explanation for some experimental results; 2) calculate the reaction rate at different temperature; 3) determine the optimum temperature for reaction.

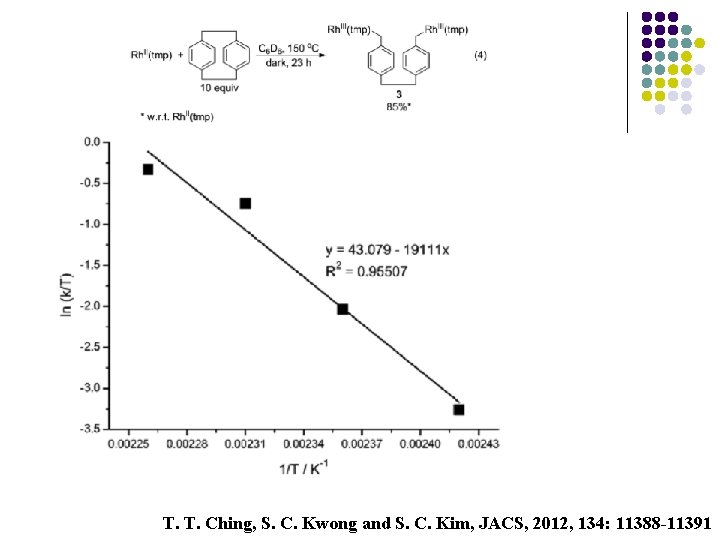
T. T. Ching, S. C. Kwong and S. C. Kim, JACS, 2012, 134: 11388 -11391
 Eyring equation and arrhenius equation
Eyring equation and arrhenius equation Eyring equation
Eyring equation Rate law units
Rate law units Order of reaction
Order of reaction Arrhenius acid base reaction
Arrhenius acid base reaction Arrhenius reaction example
Arrhenius reaction example Arrhenius equation
Arrhenius equation Arrhenius equation two point form
Arrhenius equation two point form 2 point arrhenius equation
2 point arrhenius equation Arrhenius equation
Arrhenius equation Ictahedron
Ictahedron Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Bomb power
Bomb power Equilibrium reaction rate
Equilibrium reaction rate First order rate law
First order rate law Molecularity of reaction
Molecularity of reaction How to calculate rate of reaction
How to calculate rate of reaction How to find the rate law
How to find the rate law How to calculate the instantaneous rate of reaction
How to calculate the instantaneous rate of reaction Rate law for first order reaction
Rate law for first order reaction Investigate a factor affecting the initial rate of reaction
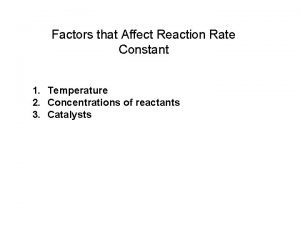
Investigate a factor affecting the initial rate of reaction Factors that affect the rate constant
Factors that affect the rate constant Factors affecting the rate of chemical reaction
Factors affecting the rate of chemical reaction Overall rate law of a reaction
Overall rate law of a reaction What is catalyst and how it affects reaction rate
What is catalyst and how it affects reaction rate