2 8 Tonicity and Osmoregulation 1 Effects of













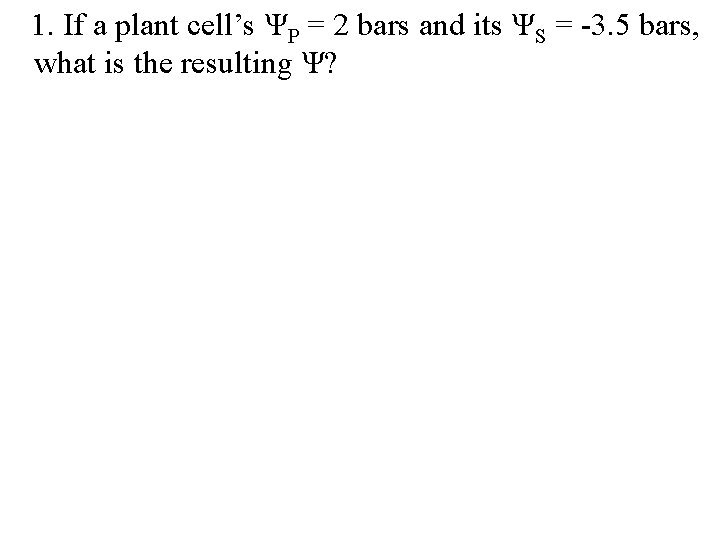
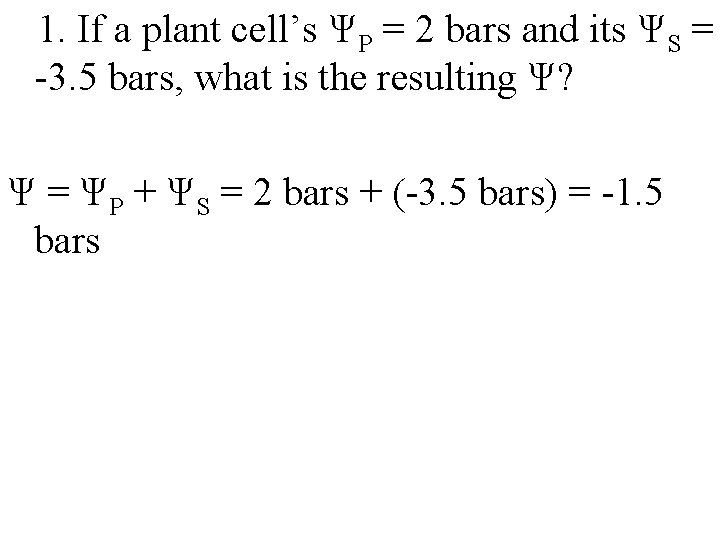








- Slides: 40

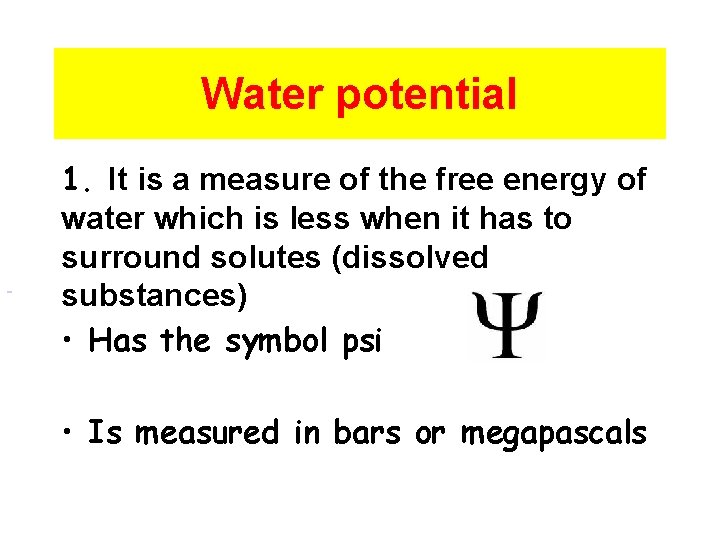
2. 8 Tonicity and Osmoregulation 1

Effects of Osmosis on Water Balance Review Question: What is a solute?

Effects of Osmosis on Water Balance Review Question: What is a solute? Answer: a substance that is dissolved in a solvent

Effects of Osmosis on Water Balance 1. Osmosis - the movement of water across a semipermeable membrane a. Water always moves from low solute concentration to high solute concentration

Effects of Osmosis on Water Balance 1. Osmosis - the movement of water across a semipermeable membrane b. This movement of molecules helps cells maintain growth and homeostasis

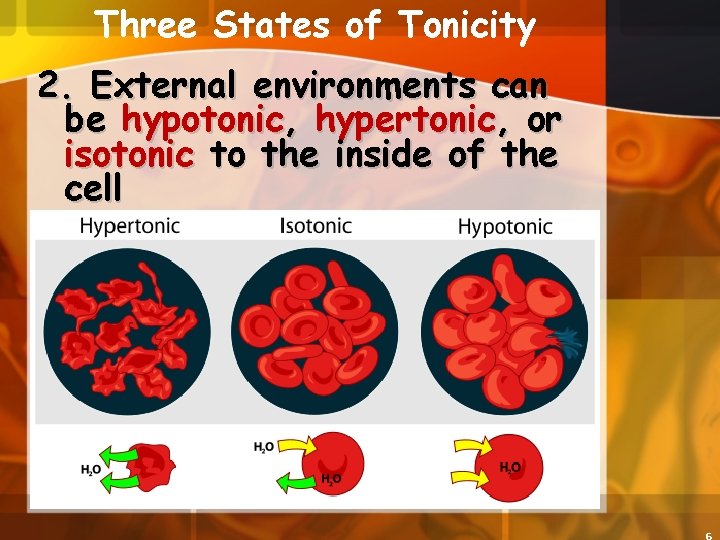
Three States of Tonicity 2. External environments can be hypotonic, hypertonic, or isotonic to the inside of the cell

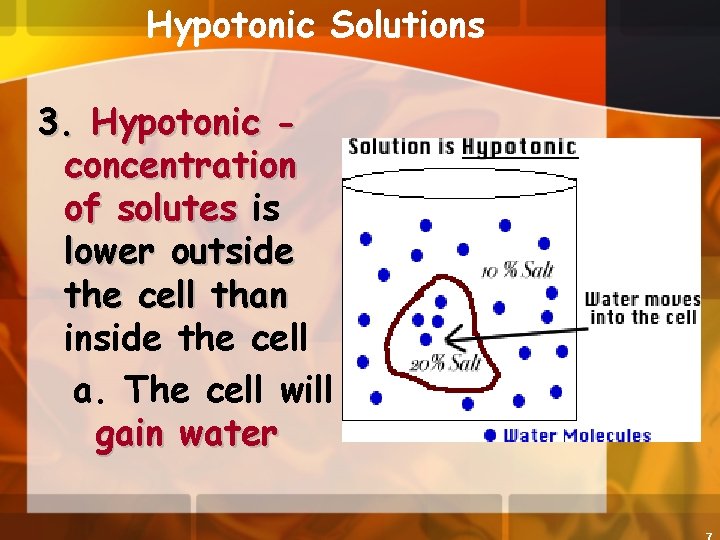
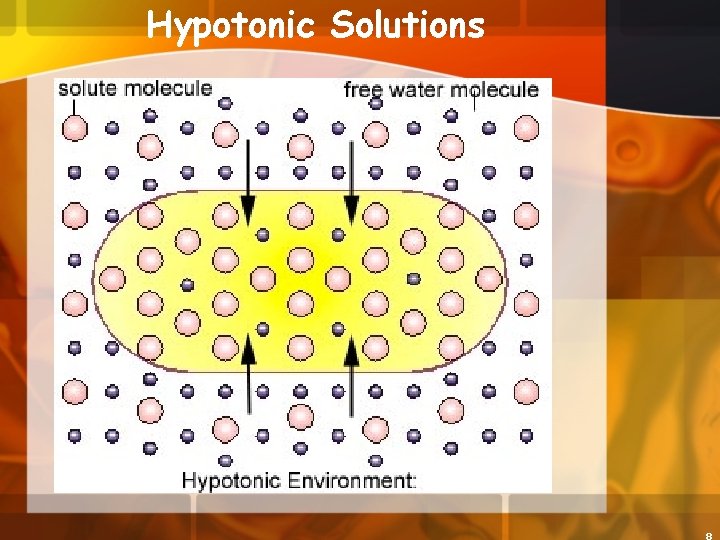
Hypotonic Solutions 3. Hypotonic concentration of solutes is lower outside the cell than inside the cell a. The cell will gain water

Hypotonic Solutions

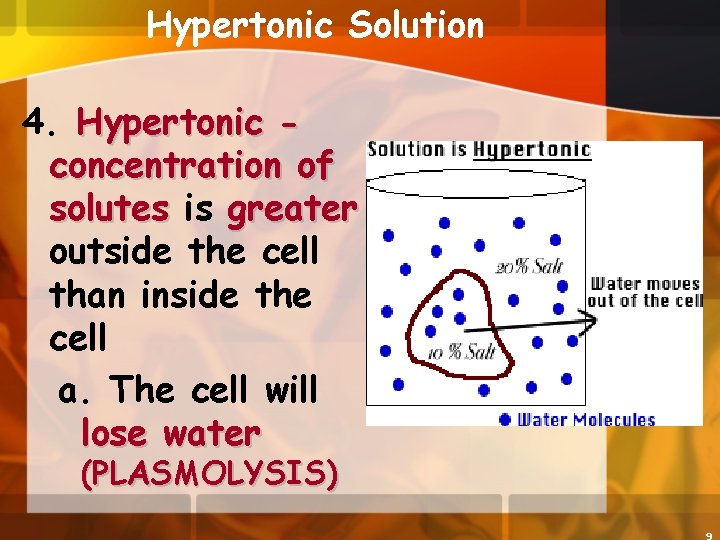
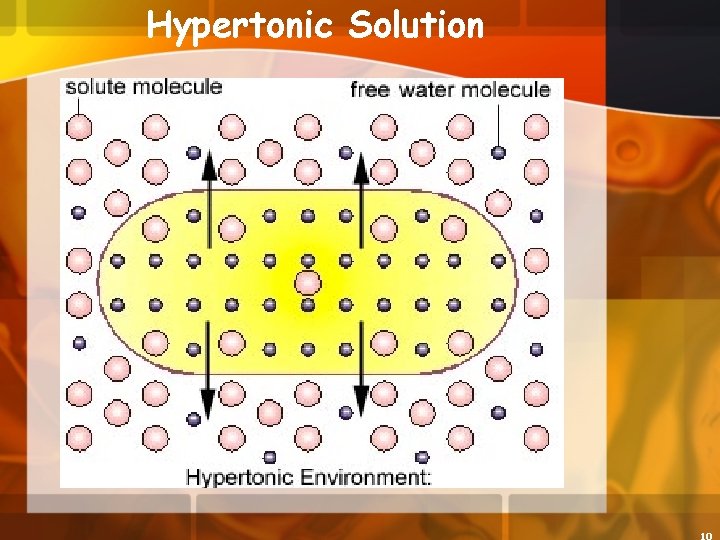
Hypertonic Solution 4. Hypertonic concentration of solutes is greater outside the cell than inside the cell a. The cell will lose water (PLASMOLYSIS)

Hypertonic Solution

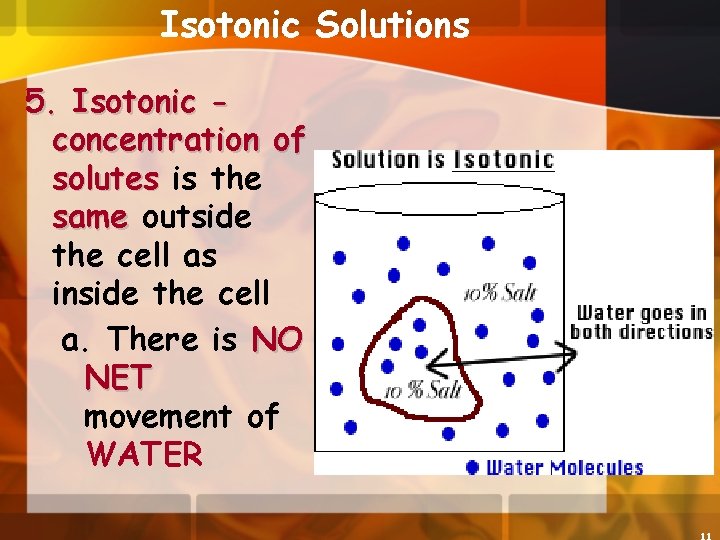
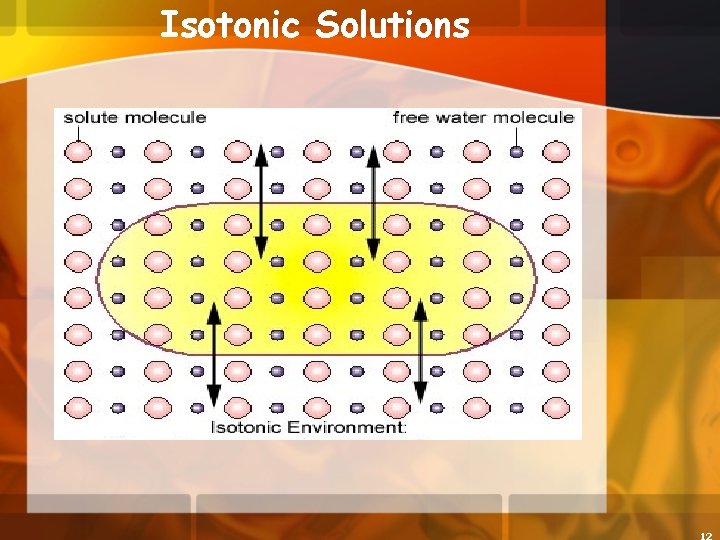
Isotonic Solutions 5. Isotonic concentration of solutes is the same outside the cell as inside the cell a. There is NO NET movement of WATER

Isotonic Solutions

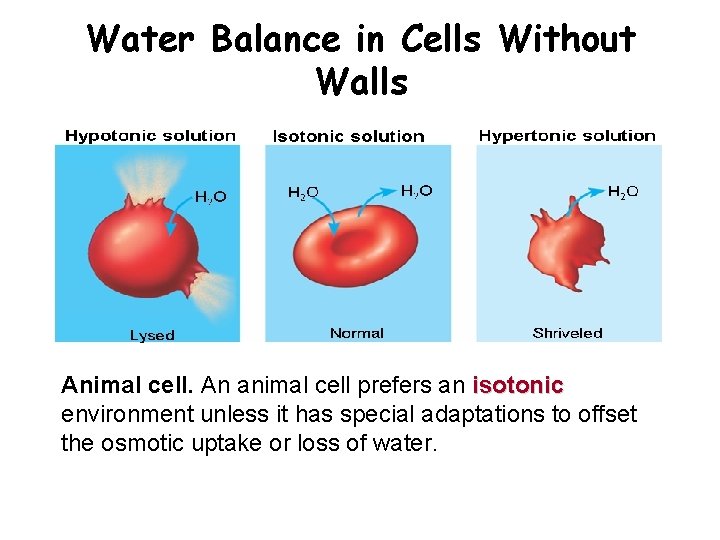
Water Balance in Cells Without Walls Animal cell. An animal cell prefers an isotonic environment unless it has special adaptations to offset the osmotic uptake or loss of water.

Water Balance of Cells with Walls 6. Osmoregulation – the maintenance of internal water balance a. allows organisms to control water and solute content

Water Balance of Cells with Walls Ex) Cell Walls help maintain water balance in plant cells Turgor pressure is the pressure of water inside a plant cell pushing outward against the cell membrane

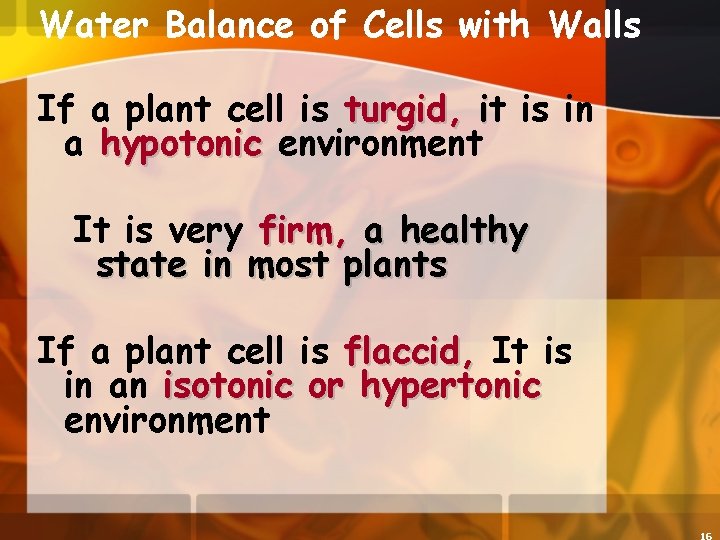
Water Balance of Cells with Walls If a plant cell is turgid, it is in a hypotonic environment It is very firm, a healthy state in most plants If a plant cell is flaccid, It is in an isotonic or hypertonic environment

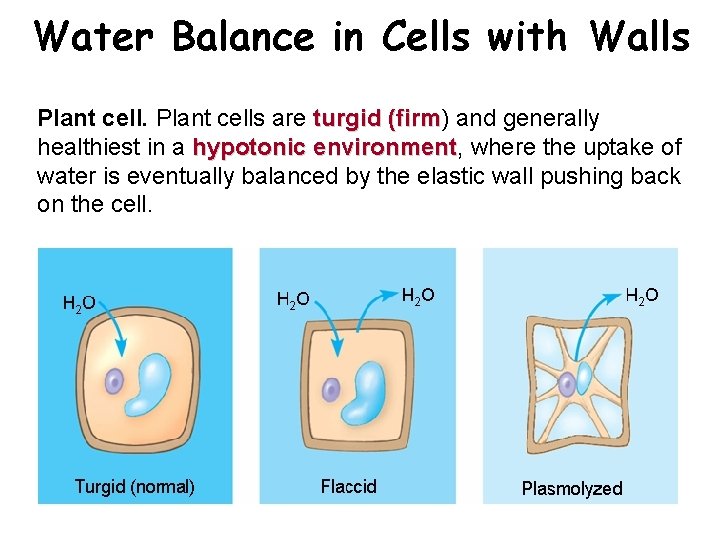
Water Balance in Cells with Walls Plant cells are turgid (firm) turgid (firm and generally healthiest in a hypotonic environment, hypotonic environment where the uptake of water is eventually balanced by the elastic wall pushing back on the cell.

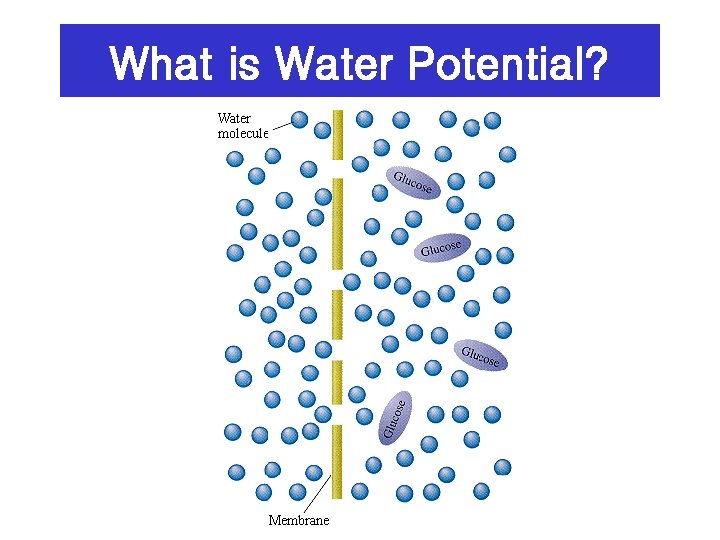
What is Water Potential?

Water potential 1. It is a measure of the free energy of water which is less when it has to surround solutes (dissolved substances) • Has the symbol psi • Is measured in bars or megapascals

Has two components: • Solute potential (also called osmotic potential) џs which is a measure of solute concentration • Pressure potential џp which is a measure of pressure on membranes/walls as water moves in or out; can be positive or negative

2. The water potential of pure water is ZERO • A. Because pure water has the highest concentration of water molecules, and thus the highest water potential, the water potential of all other solutions must be lower than zero i. e. negative.

Pure water: = 0

3. Adding solute decreases water potential! • The more solute there is present in a solution the more negative it becomes. • So, solute potential will be a negative number if not pure water. 4. So hypertonic solutions have negative solute potentials.

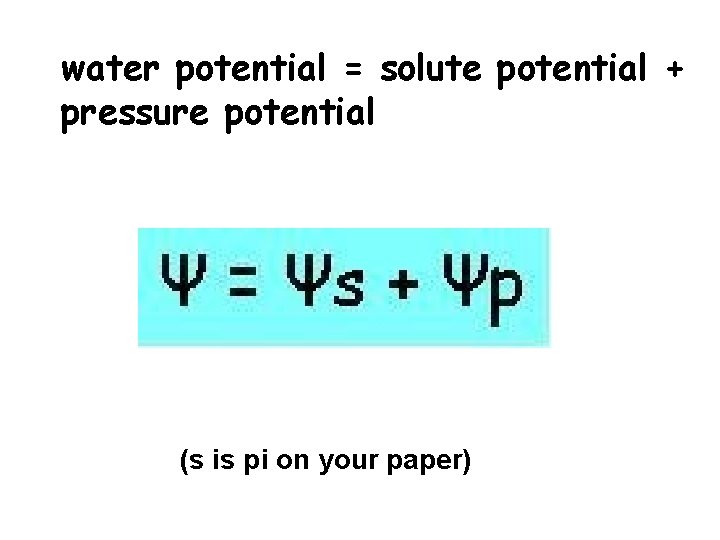
water potential = solute potential + pressure potential (s is pi on your paper)

5. Water moves from areas of higher water potential to areas of lower water potential (i. e. towards the more negative, concentrated region). water always "falls" from a high to a low water potential

This will occur until the water potential inside the cell equals the water potential outside of the cell.

If this makes no sense whatsoever the key information to learn is: • The equation given • the water potential of pure water is zero • water moves from areas of higher water potential to areas of lower water potential (i. e. towards the more negative region)
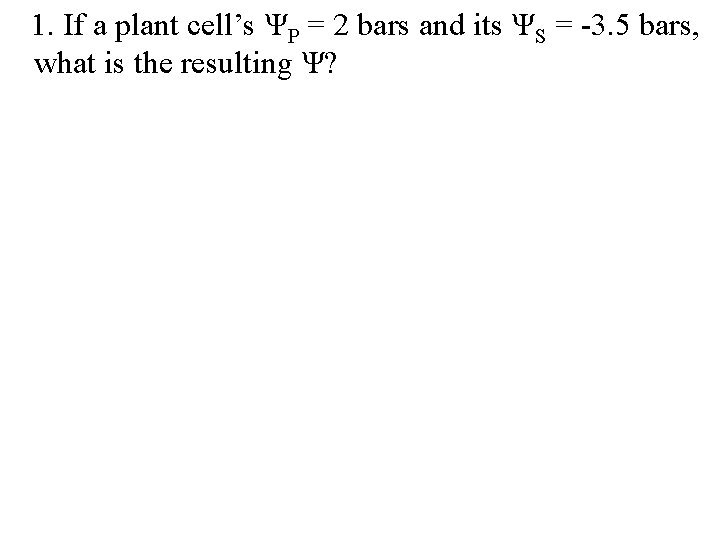
1. If a plant cell’s ΨP = 2 bars and its ΨS = -3. 5 bars, what is the resulting Ψ?
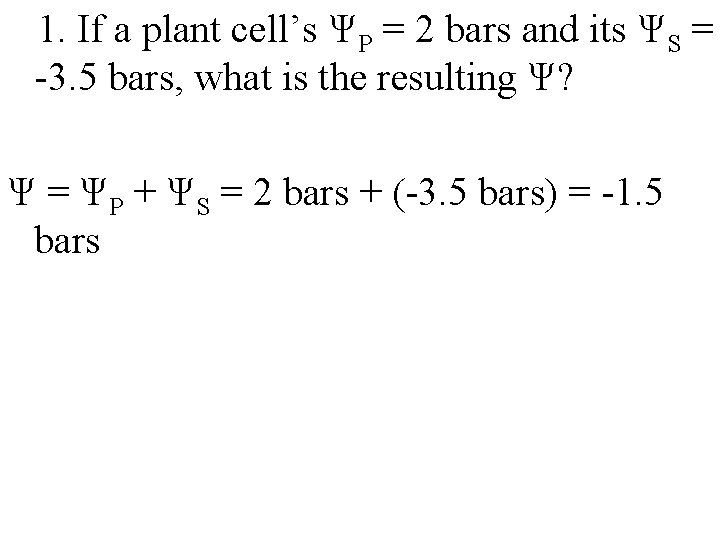
1. If a plant cell’s ΨP = 2 bars and its ΨS = -3. 5 bars, what is the resulting Ψ? Ψ = ΨP + ΨS = 2 bars + (-3. 5 bars) = -1. 5 bars

2. A cell is placed in an isotonic solution. The solute potential is found to be 2. 1 MPa. What is the water potential of the cell?

2. A cell is placed in an isotonic solution. The solute potential is found to be 2. 1 MPa. What is the water potential of the cell? Ψ = ΨP + ΨS = 0 MPa + (-2. 1 MPa) = -2. 1 MPa

3. A cell with a water potential of 2 MPa is placed in a solution with a potential of -4 MPa. Which direction will the water move?

3. A cell with a water potential of 2 MPa is placed in a solution with a potential of -4 MPa. Which direction will the water move? Water always moves toward lower water potential, so the water will move from the cell to the solution.

What if you aren’t given solute potential?

What if you aren’t given solute potential? • You can find it!

What if you aren’t given solute potential? • Another equation solute potential = -i. CRT I = ionization constant (1 for sucrose) C = molar concentration R = pressure constant (0. 0831 liter/bars/mole 0 K) T = temperature Kelvin (273 + C) Units will cancel out to equal bars.

The value for Ψ in root tissue was found to be 3. 3 bars. If you place the root tissue in a 0. 1 M solution of sucrose at 22°C in an open beaker, what is the Ψ of the solution, and in which direction would the net flow of water be? ΨS = -i. CRT

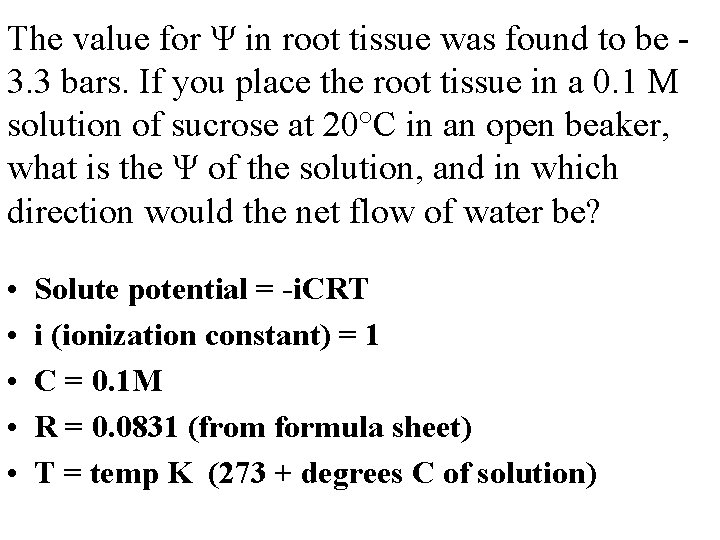
The value for Ψ in root tissue was found to be 3. 3 bars. If you place the root tissue in a 0. 1 M solution of sucrose at 20°C in an open beaker, what is the Ψ of the solution, and in which direction would the net flow of water be? • • • Solute potential = -i. CRT i (ionization constant) = 1 C = 0. 1 M R = 0. 0831 (from formula sheet) T = temp K (273 + degrees C of solution)

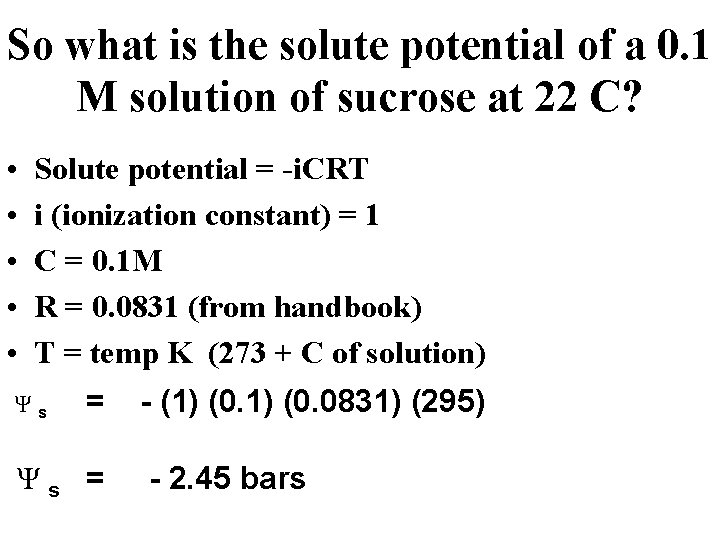
So what is the solute potential of a 0. 1 M solution of sucrose at 22 C? • • • Solute potential = -i. CRT i (ionization constant) = 1 C = 0. 1 M R = 0. 0831 (from handbook) T = temp K (273 + C of solution) Ψ s = - (1) (0. 0831) (295) Ψ s = - 2. 45 bars

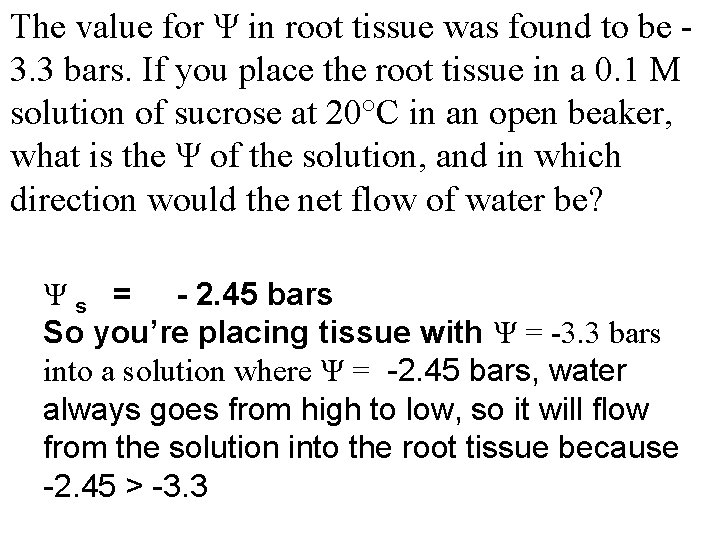
The value for Ψ in root tissue was found to be 3. 3 bars. If you place the root tissue in a 0. 1 M solution of sucrose at 20°C in an open beaker, what is the Ψ of the solution, and in which direction would the net flow of water be? Ψ s = - 2. 45 bars So you’re placing tissue with Ψ = -3. 3 bars into a solution where Ψ = -2. 45 bars, water always goes from high to low, so it will flow from the solution into the root tissue because -2. 45 > -3. 3