Tonicity HyperHypoIsotonic Osmosis Just like other particles water







- Slides: 15

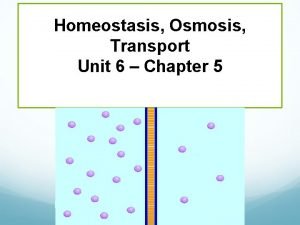
Tonicity Hyper-/Hypo-/Isotonic

Osmosis • Just like other particles, water is free to diffuse in and out of a cell DOWN it’s concentration gradient. • …through channel proteins • Therefore, this process, called osmosis, is the facilitated diffusion of FREE water molecules across a membrane.

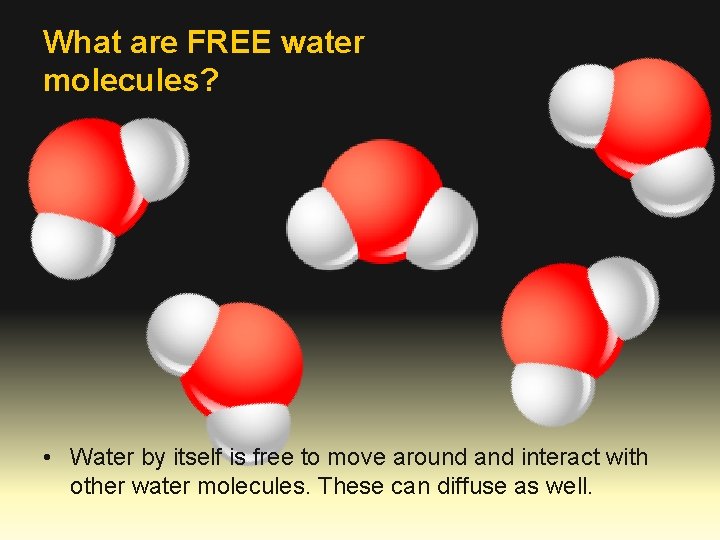
What are FREE water molecules? • Water by itself is free to move around and interact with other water molecules. These can diffuse as well.

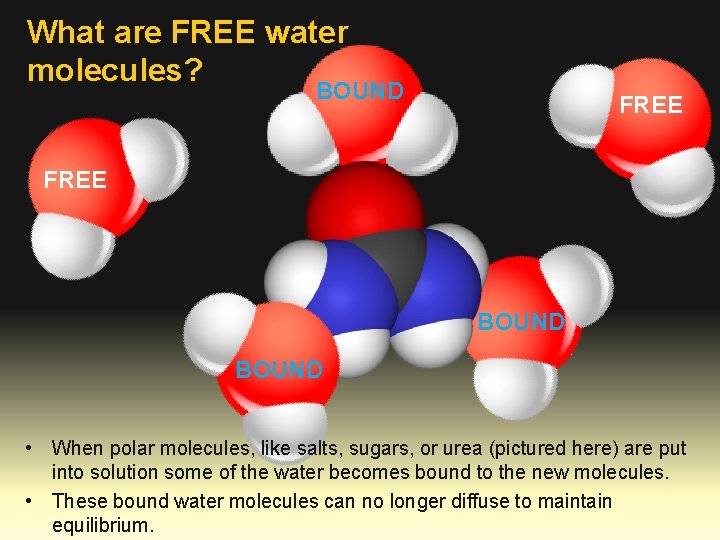
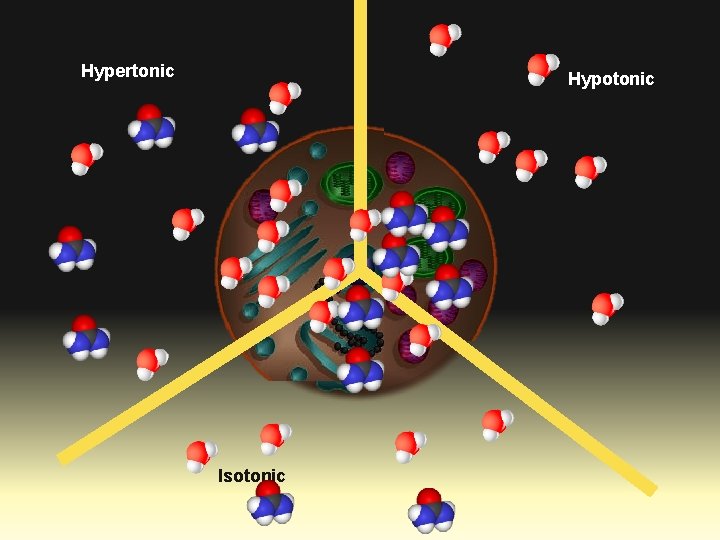
What are FREE water molecules? BOUND FREE BOUND • When polar molecules, like salts, sugars, or urea (pictured here) are put into solution some of the water becomes bound to the new molecules. • These bound water molecules can no longer diffuse to maintain equilibrium.

How is Equilibrium Achieved? MEMBRANE PORE

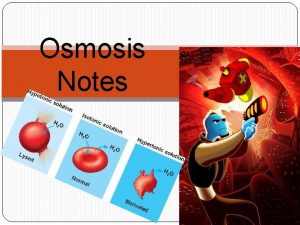
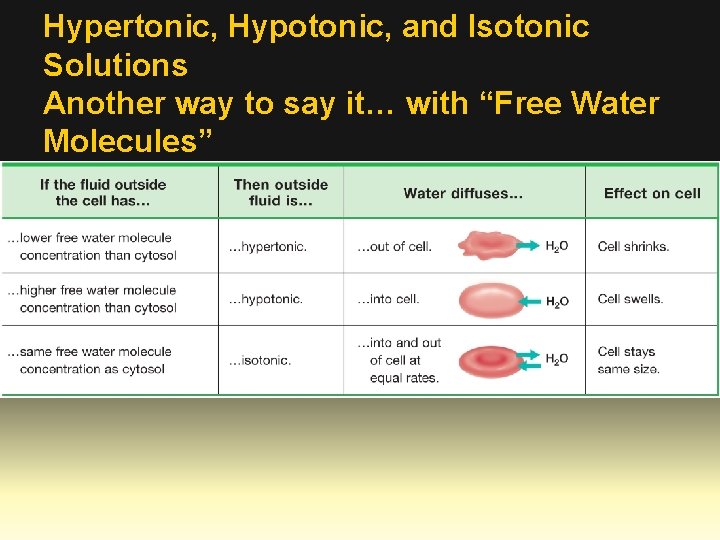
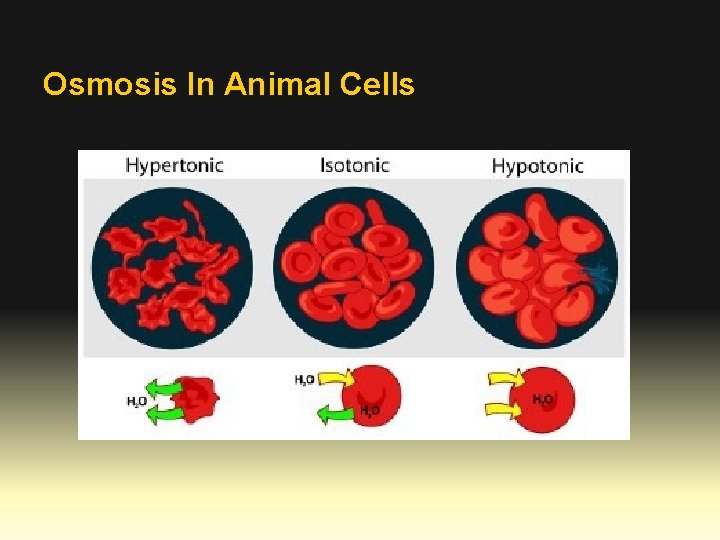
Osmosis • The direction of water movement in a cell depends on the concentration of the cell’s outside environment. • If the solution is hypertonic, or has a higher solute concentration than the cytoplasm does, water moves out of the cell. The cell loses water and shrinks. • If the solution is hypotonic, or has a lower solute concentration than the cytoplasm does, water moves into the cell. The cell gains water and expands in size. • If the solution is isotonic, or has the same solute concentration that the cytoplasm does, water diffuses into and out of the cell at equal rates. The cell stays the same size.

how_osmosis_works. html Osmosis/ How the Environment Changes • When ions and polar substances dissolve in water, they attract and bind some water molecules. The remaining water molecules are free to move around. • If a concentration gradient exists across a membrane for solutes, a concentration gradient also exists across the membrane for free water molecules. • Osmosis occurs as free water molecules move down their concentration gradient into the solution that has the lower concentration of free water molecules.

Hypertonic, Hypotonic, and Isotonic Solutions Another way to say it… with “Free Water Molecules”

Hypertonic Hypotonic Isotonic

Osmosis In Animal Cells

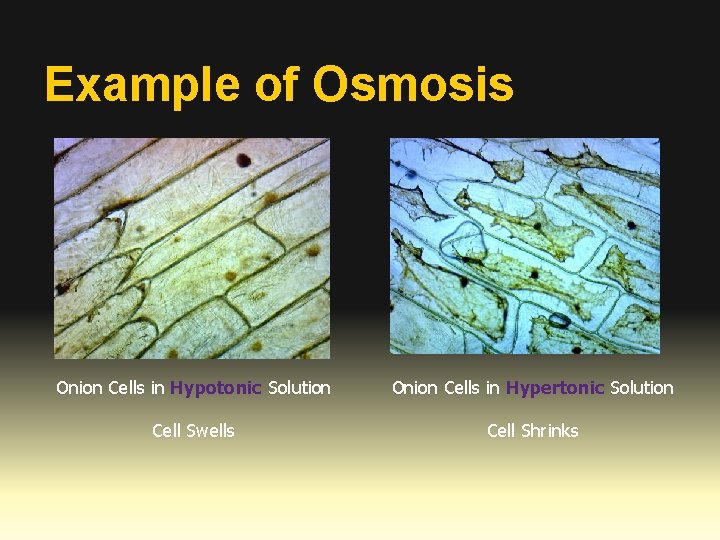
Example of Osmosis Onion Cells in Hypotonic Solution Onion Cells in Hypertonic Solution Cell Swells Cell Shrinks

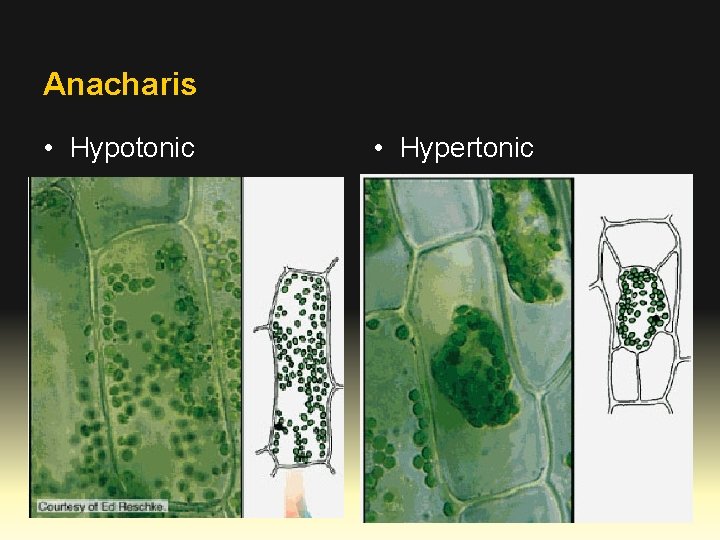
Anacharis • Hypotonic • Hypertonic

Questions? ? ? • Now, get into groups and complete the practice problems.

Questions? ? ? • Now get into your groups, collect your final data (volume & mass), and collaborate on what you witnessed. • Use the new “Day 2” procedures handed out today. • At the end of class we will discuss some of your determinations.

Analysis Questions • What does your bar graph look like? • What do you need on Monday? – Poster board (1 per group) – Analysis graphs (rough draft)
 Water and water and water water
Water and water and water water A piece of wood floats on water
A piece of wood floats on water Osmotic potential
Osmotic potential Osmosis water and salt
Osmosis water and salt Particles attract each other
Particles attract each other Why does ethanol look like water but behave more like wood?
Why does ethanol look like water but behave more like wood? Christian schill
Christian schill Point like particles
Point like particles Gas like mixture of charged particles
Gas like mixture of charged particles Rewrite the final six lines of romeo and juliet
Rewrite the final six lines of romeo and juliet What is tonicity
What is tonicity Tonicity شرح
Tonicity شرح Tonicity
Tonicity Tonicity of fluid
Tonicity of fluid Osmolarity and osmolality
Osmolarity and osmolality Osmolality vs tonicity
Osmolality vs tonicity