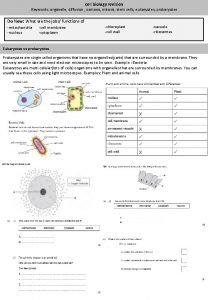
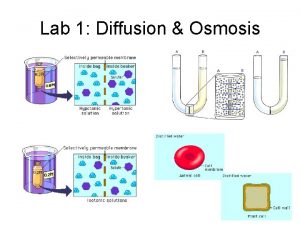
Osmosis Osmosis is the diffusion of water molecules















- Slides: 24

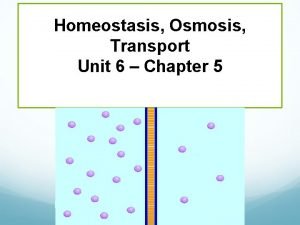
Osmosis

Osmosis… • …is the diffusion of water molecules • …happens across a semi-permeable membrane

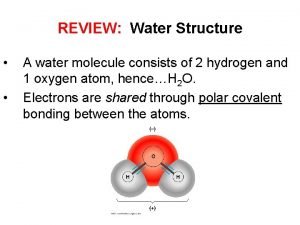
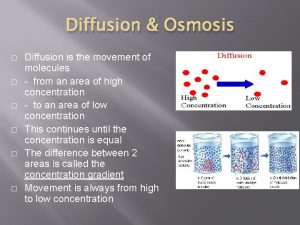
ONLY WATER – Water is a small but extremely important molecule that makes up most of the liquid part of the cytoplasm in living things. – Deals ONLY with the diffusion of WATER – The molecules (in this case, water - not solute molecules) will tend to move from an area of high (water) concentration to an area of low(water)concentration until equilibrium is reached.


OSMOSIS: FACILITATED DIFFUSION OF WATER ACROSS A CELL MEMBRANE Why would water molecules normally have a hard time getting across the cell membrane? The inside of a cell’s lipid bilayer is hydrophobic (water hating) Click me!

Aquaporins • Most cells have special water channel proteins – Known as – Aquaporins • Allow H 2 O to pass right through them by facilitated diffusion. • This EXTREMELY important process is = OSMOSIS

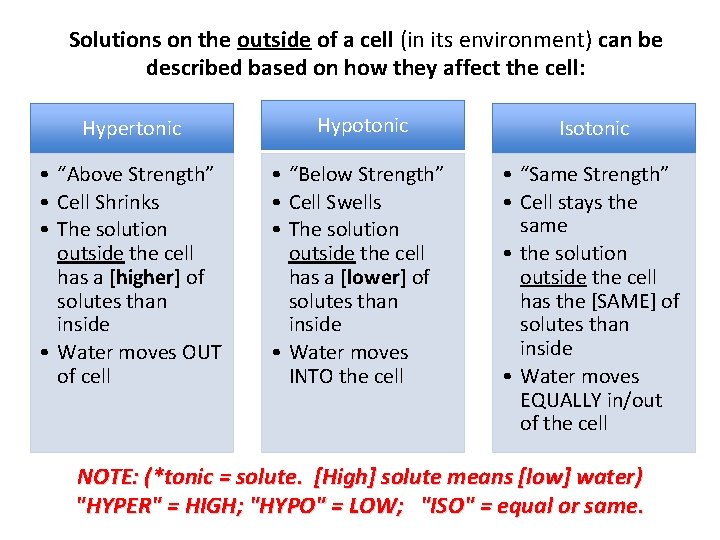
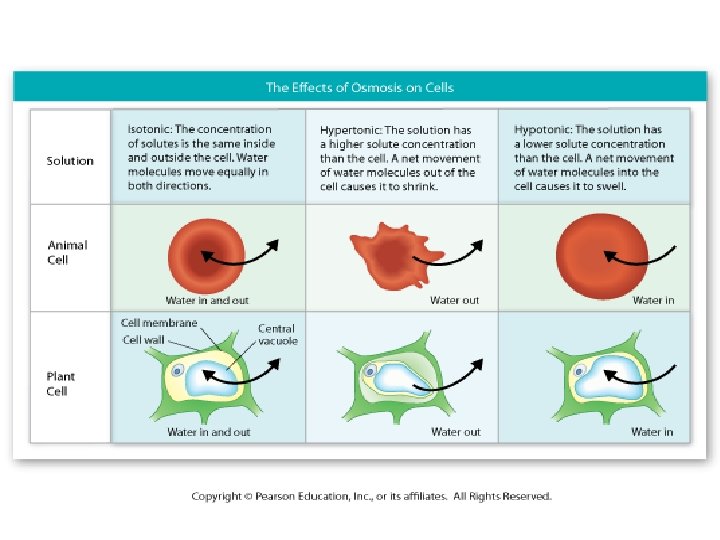
• By knowing the concentrations of solute and solvent on the inside and outside of a cell, we can predict the direction of osmosis and the result on the cell. • Solutions on the outside of a cell can be described based on how they affect the cell – hyp. ERtonic – hyp. Otonic – isotonic

Solutions on the outside of a cell (in its environment) can be described based on how they affect the cell: Hypertonic Hypotonic Isotonic • “Above Strength” • Cell Shrinks • The solution outside the cell has a [higher] of solutes than inside • Water moves OUT of cell • “Below Strength” • Cell Swells • The solution outside the cell has a [lower] of solutes than inside • Water moves INTO the cell • “Same Strength” • Cell stays the same • the solution outside the cell has the [SAME] of solutes than inside • Water moves EQUALLY in/out of the cell NOTE: (*tonic = solute. [High] solute means [low] water) "HYPER" = HIGH; "HYPO" = LOW; "ISO" = equal or same.

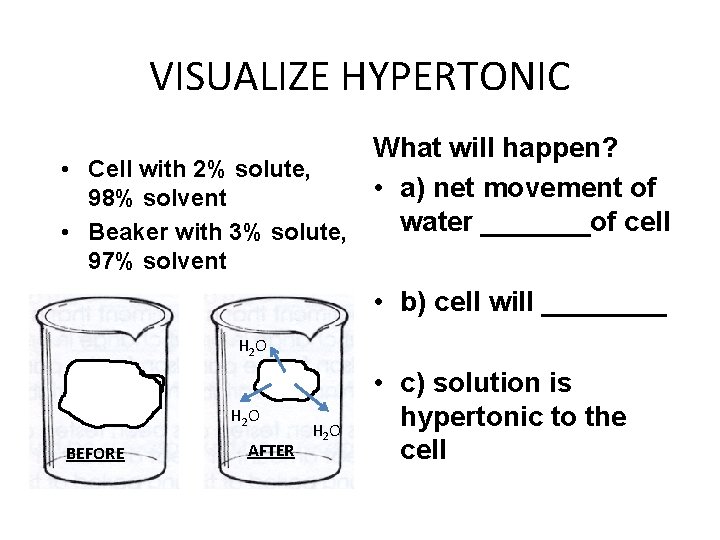
VISUALIZE HYPERTONIC What will happen? • Cell with 2% solute, • a) net movement of 98% solvent water _______of cell • Beaker with 3% solute, 97% solvent • b) cell will ____ H 2 O BEFORE AFTER H 2 O • c) solution is hypertonic to the cell

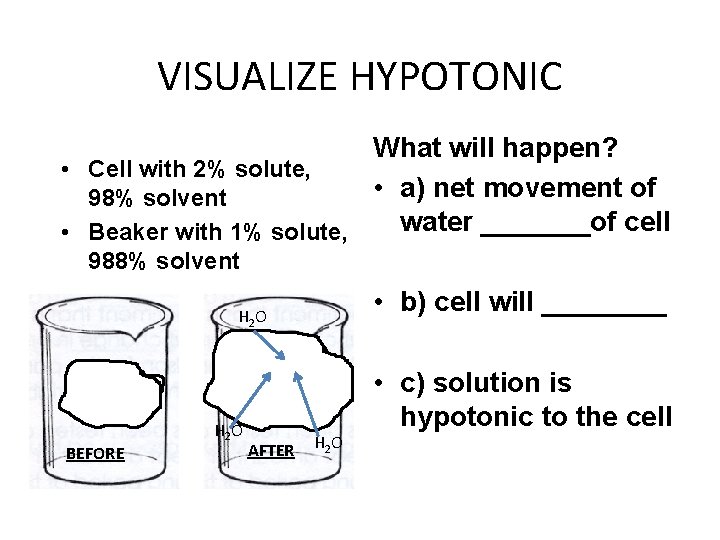
VISUALIZE HYPOTONIC What will happen? • Cell with 2% solute, • a) net movement of 98% solvent water _______of cell • Beaker with 1% solute, 988% solvent • b) cell will ____ H 2 O BEFORE H 2 O AFTER H 2 O • c) solution is hypotonic to the cell

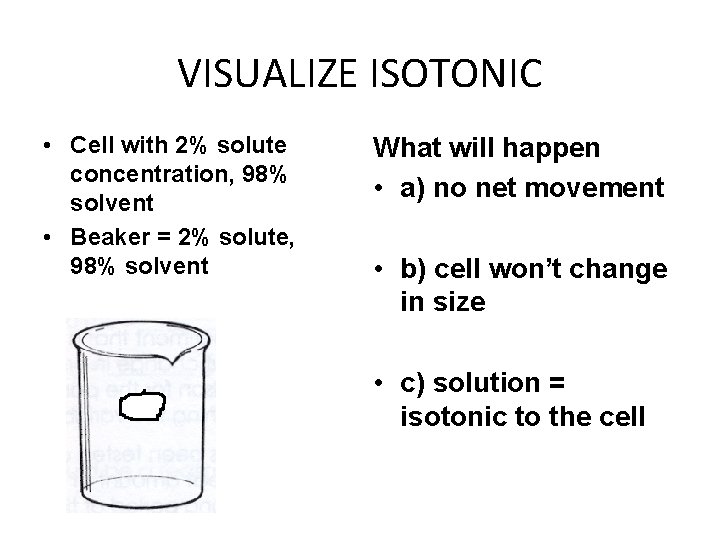
VISUALIZE ISOTONIC • Cell with 2% solute concentration, 98% solvent • Beaker = 2% solute, 98% solvent What will happen • a) no net movement • b) cell won’t change in size • c) solution = isotonic to the cell

Common mistakes when discussing hyper-, hypo-, and isotonic solutions • The solutions are named for the concentrations of the SOLUTES • The substance that moves to balance the solute concentration is the WATER • The solutes to not “pull” or “suck” the water across the membrane – the water simply diffuses from where it is in high concentration to low concentration

Solute and solvent concentrations can be expressed as percentages of the entire solution. • When added together, the solute and solvent concentrations must equal 100%. • A solution with a 10 % solute concentration has a 90% solvent concentration.

Let’s do some math! • What is the solvent concentration of a solution with a 3% concentration of solute? • What is the solvent concentration of a solution with a 15% concentration of glucose? • What is the solute concentration of a solution with 98% solvent? • What is the solute concentration of a solution with 75% water?

OSMOTIC PRESSURE Driven by differences in solute concentration, the net movement of water into or out of a cell produces a force known as osmotic pressure

Almost always hypertonic… • Because cells contain a variety of solutes such as: – sugars, proteins, salts, etc. – they are almost always hypertonic • (*the environment = HYPOtonic!) to fresh water; • as a result, a typical cell exposed to fresh water will tend to swell up quickly from the entering water. • This may in fact cause an animal cell to swell like an overinflated balloon.

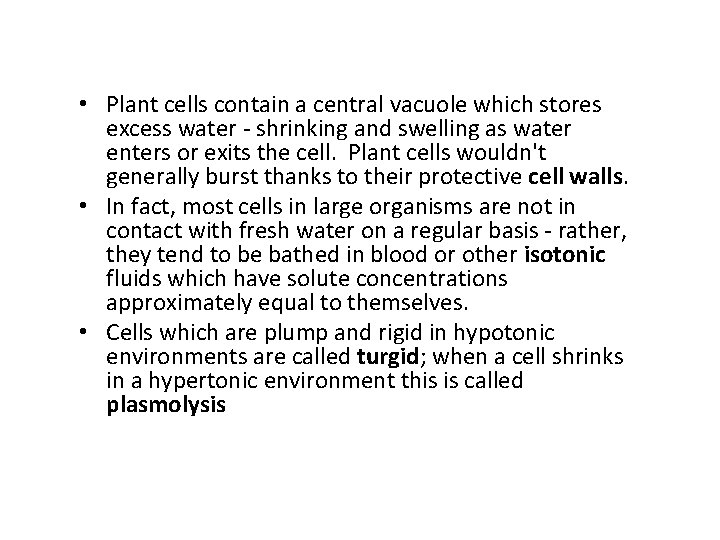
• Plant cells contain a central vacuole which stores excess water - shrinking and swelling as water enters or exits the cell. Plant cells wouldn't generally burst thanks to their protective cell walls. • In fact, most cells in large organisms are not in contact with fresh water on a regular basis - rather, they tend to be bathed in blood or other isotonic fluids which have solute concentrations approximately equal to themselves. • Cells which are plump and rigid in hypotonic environments are called turgid; when a cell shrinks in a hypertonic environment this is called plasmolysis

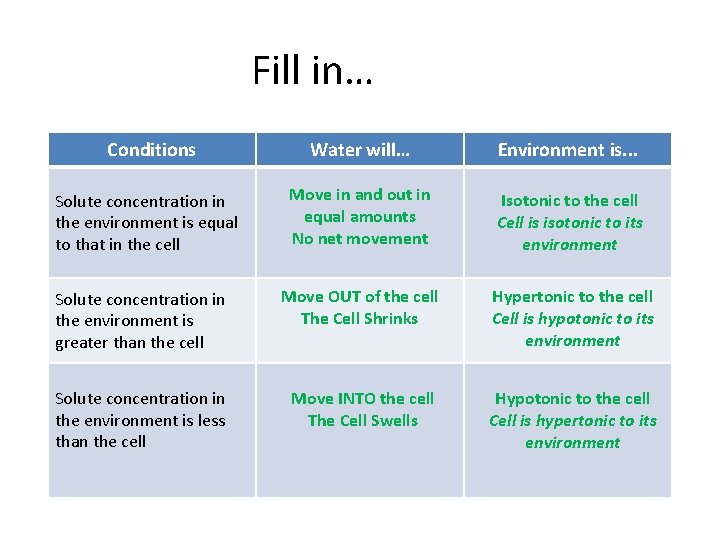
Fill in… Conditions Water will… Environment is. . . Solute concentration in the environment is equal to that in the cell Move in and out in equal amounts No net movement Isotonic to the cell Cell is isotonic to its environment Solute concentration in the environment is greater than the cell Move OUT of the cell The Cell Shrinks Hypertonic to the cell Cell is hypotonic to its environment Solute concentration in the environment is less than the cell Move INTO the cell The Cell Swells Hypotonic to the cell Cell is hypertonic to its environment

Some more practice!

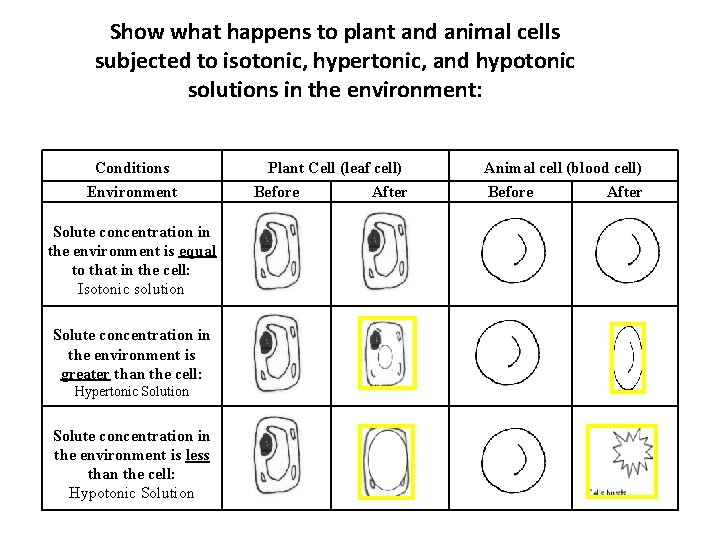
Show what happens to plant and animal cells subjected to isotonic, hypertonic, and hypotonic solutions in the environment: Conditions Environment Solute concentration in the environment is equal to that in the cell: Isotonic solution Solute concentration in the environment is greater than the cell: Hypertonic Solution Solute concentration in the environment is less than the cell: Hypotonic Solution Plant Cell (leaf cell) Before After Animal cell (blood cell) Before After


APPLY what you have learned about osmosis… • Why do doctor’s use a saline solution in an IV drip?

APPLY what you have learned about osmosis… • Why would salt kill plants?

APPLY what you have learned about osmosis… • Why do restaurants put out free salty snacks such as peanuts, pretzels or chips?
 Water and water and water water
Water and water and water water Pinocytosis vs phagocytosis
Pinocytosis vs phagocytosis Facilitated diffusion vs osmosis
Facilitated diffusion vs osmosis Diffusion osmosis
Diffusion osmosis Receptor - mediated endocytosis
Receptor - mediated endocytosis Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Swabt
Swabt Relocation diffusion vs expansion diffusion
Relocation diffusion vs expansion diffusion Flaccid turgid and plasmolysis
Flaccid turgid and plasmolysis Osmosis water and salt
Osmosis water and salt Is h2o a polar molecule
Is h2o a polar molecule Intermolecular forces in water
Intermolecular forces in water A solid compound that contains water molecules
A solid compound that contains water molecules Properties of water
Properties of water Bond between water molecules
Bond between water molecules Molecul
Molecul Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Thang điểm glasgow
Thang điểm glasgow Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất