12 9 Rate and Regioselectivity in Electrophilic Aromatic














- Slides: 43

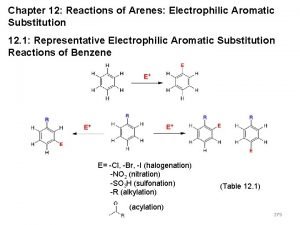
12. 9 Rate and Regioselectivity in Electrophilic Aromatic Substitution A substituent already present on the ring can affect both the rate and regioselectivity of electrophilic aromatic substitution.

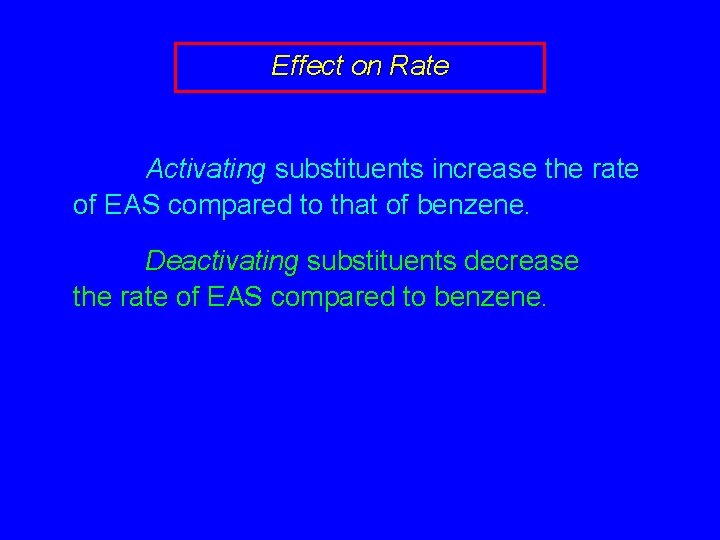
Effect on Rate Activating substituents increase the rate of EAS compared to that of benzene. Deactivating substituents decrease the rate of EAS compared to benzene.

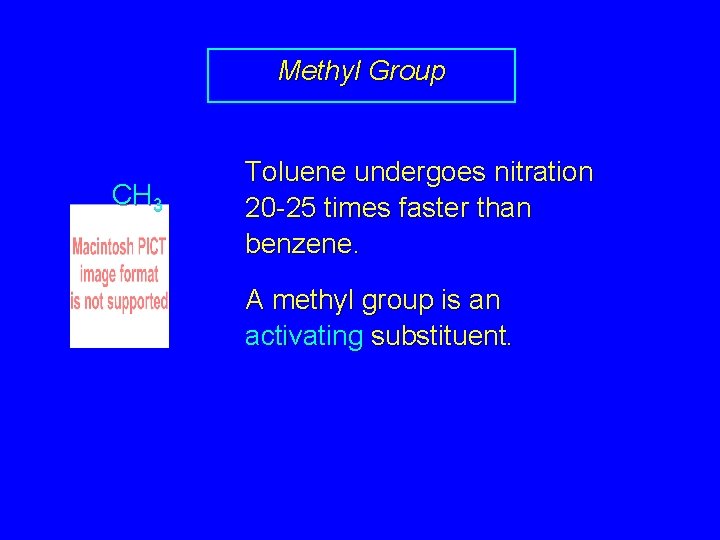
Methyl Group CH 3 Toluene undergoes nitration 20 -25 times faster than benzene. A methyl group is an activating substituent.

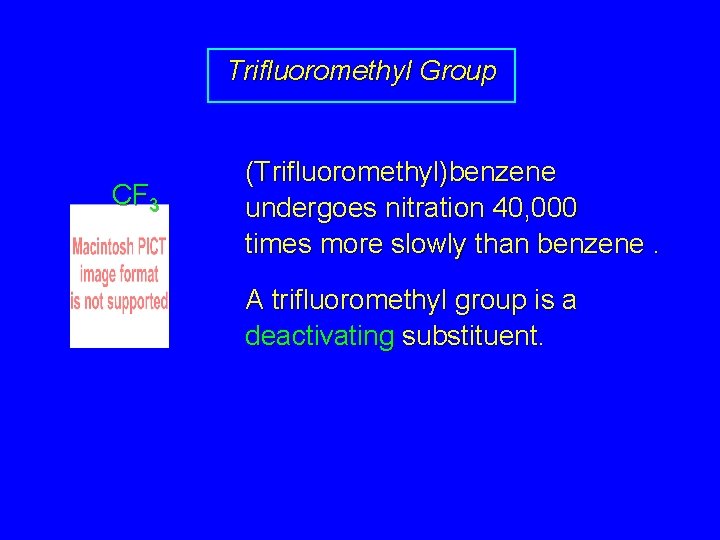
Trifluoromethyl Group CF 3 (Trifluoromethyl)benzene undergoes nitration 40, 000 times more slowly than benzene. A trifluoromethyl group is a deactivating substituent.

Effect on Regioselectivity Ortho-para directors direct an incoming electrophile to positions ortho and/or para to themselves. Meta directors direct an incoming electrophile to positions meta to themselves.

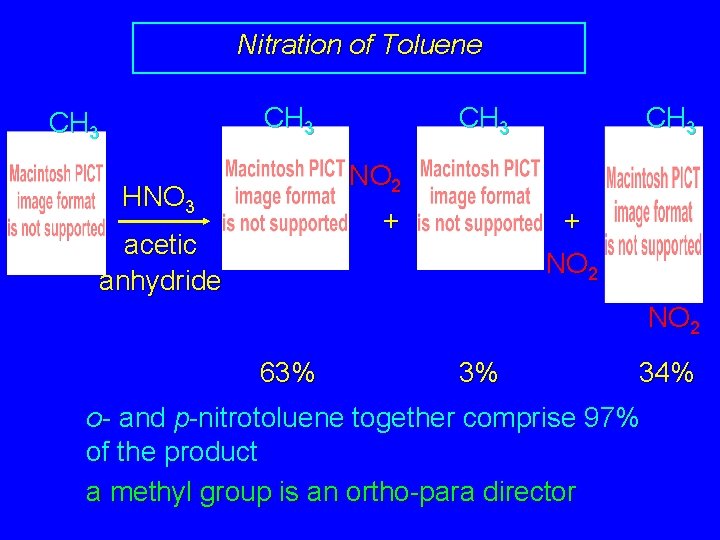
Nitration of Toluene CH 3 NO 2 HNO 3 + acetic anhydride + NO 2 63% 3% 34% o- and p-nitrotoluene together comprise 97% of the product a methyl group is an ortho-para director

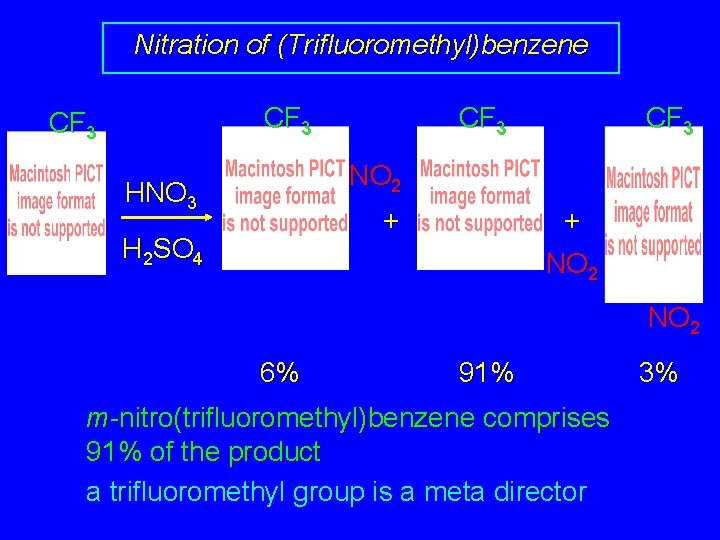
Nitration of (Trifluoromethyl)benzene CF 3 NO 2 HNO 3 + H 2 SO 4 + NO 2 6% 91% m-nitro(trifluoromethyl)benzene comprises 91% of the product a trifluoromethyl group is a meta director 3%

12. 10 Rate and Regioselectivity in the Nitration of Toluene

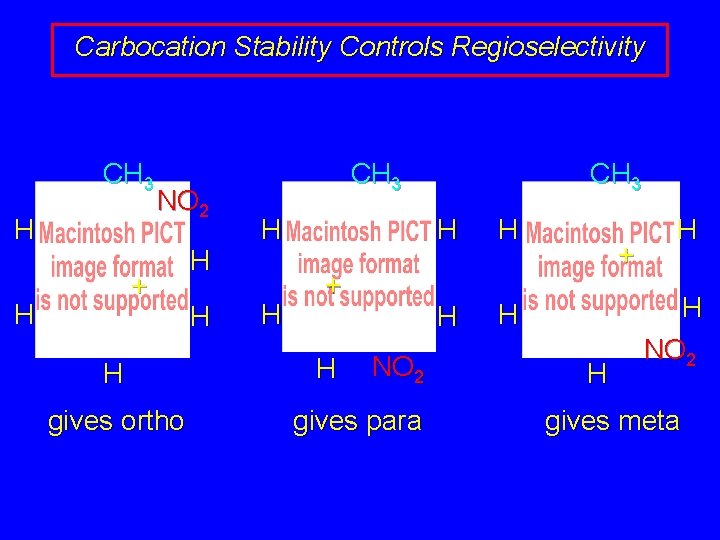
Carbocation Stability Controls Regioselectivity CH 3 H NO 2 + H H gives ortho H H CH 3 H H + H NO 2 gives para CH 3 H H + H H H NO 2 gives meta

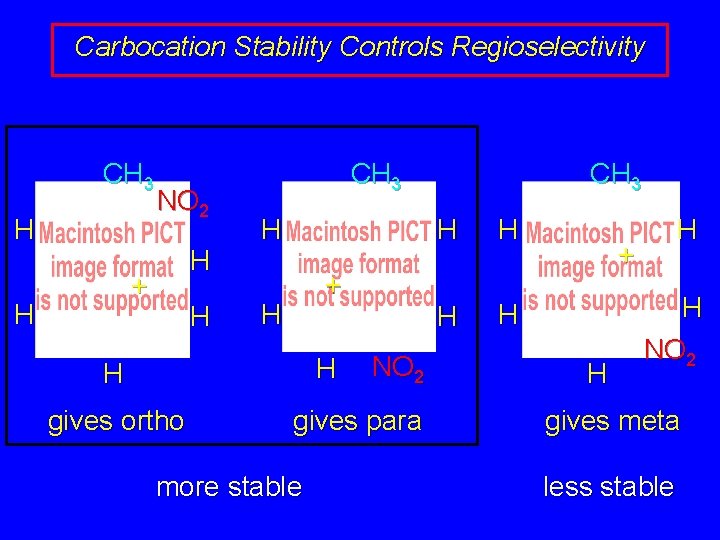
Carbocation Stability Controls Regioselectivity CH 3 H NO 2 H + H H CH 3 H + H H H gives ortho NO 2 gives para more stable CH 3 H H H + H H NO 2 gives meta less stable

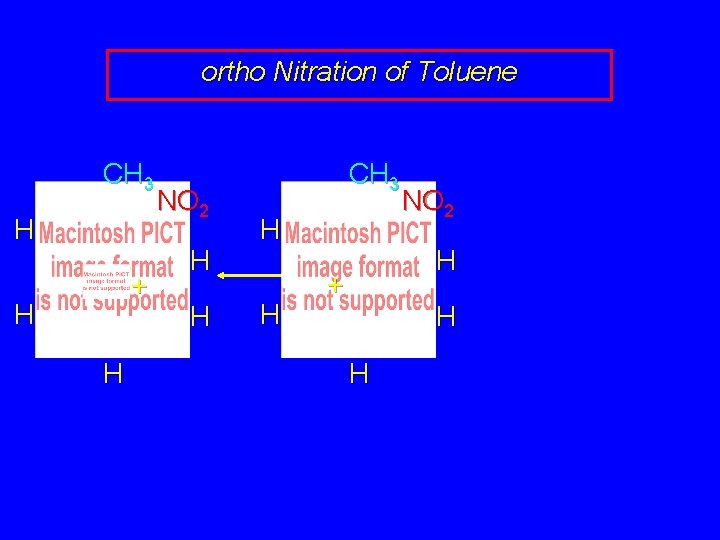
ortho Nitration of Toluene CH 3 H + H H NO 2 H H

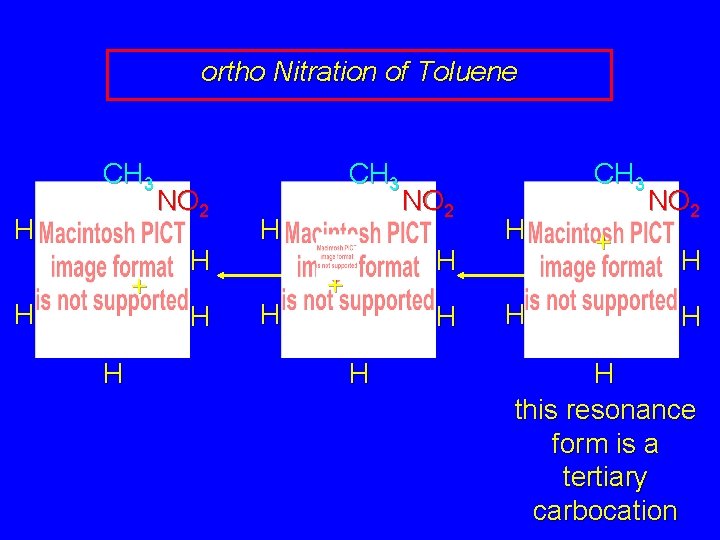
ortho Nitration of Toluene CH 3 H + H H NO 2 H H CH 3 H H NO 2 H + H H

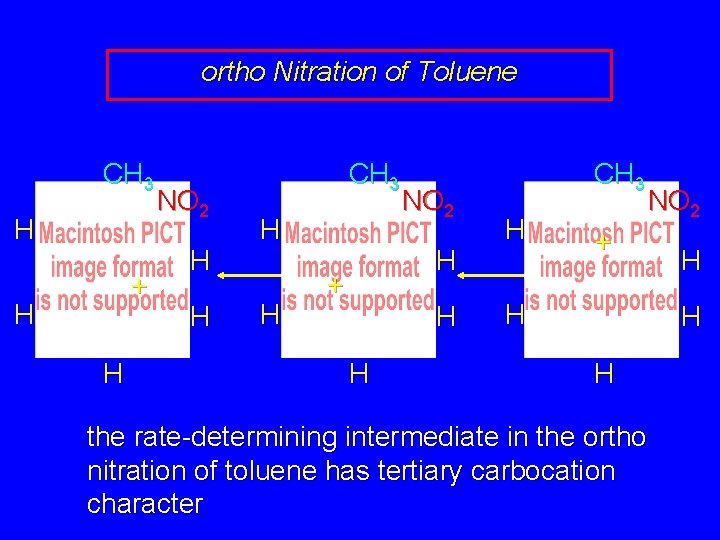
ortho Nitration of Toluene CH 3 H + H H NO 2 H H CH 3 H H NO 2 H + H H CH 3 H H + NO 2 H H H this resonance form is a tertiary carbocation

ortho Nitration of Toluene CH 3 H + H H NO 2 H H CH 3 H H NO 2 H + H H CH 3 H NO 2 + H H the rate-determining intermediate in the ortho nitration of toluene has tertiary carbocation character

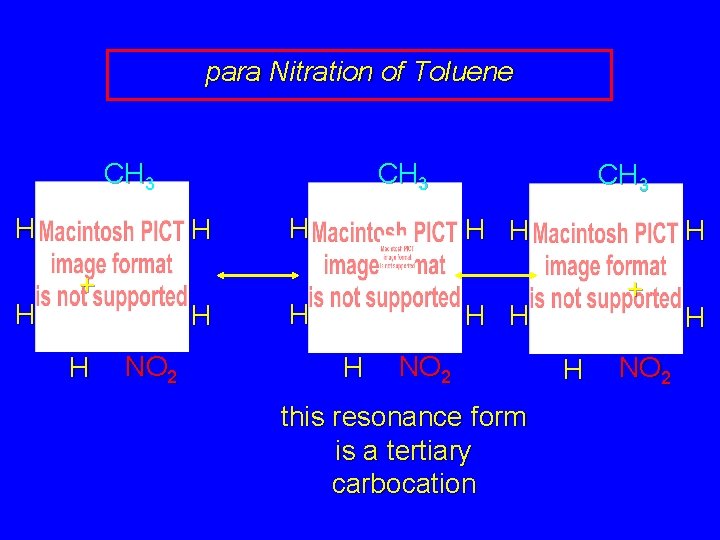
para Nitration of Toluene CH 3 H H H + H H NO 2

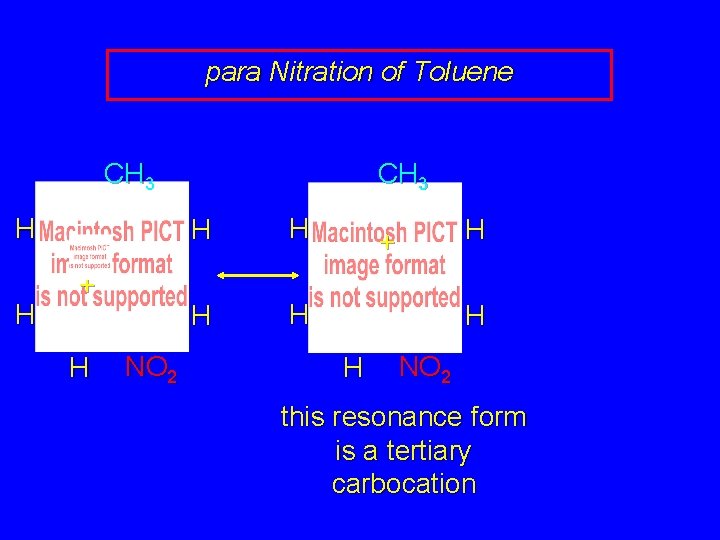
para Nitration of Toluene CH 3 H H + H NO 2 CH 3 H H H + H H NO 2 this resonance form is a tertiary carbocation

para Nitration of Toluene CH 3 H H CH 3 H + H H NO 2 H CH 3 H H + H H H NO 2 this resonance form is a tertiary carbocation H NO 2 H

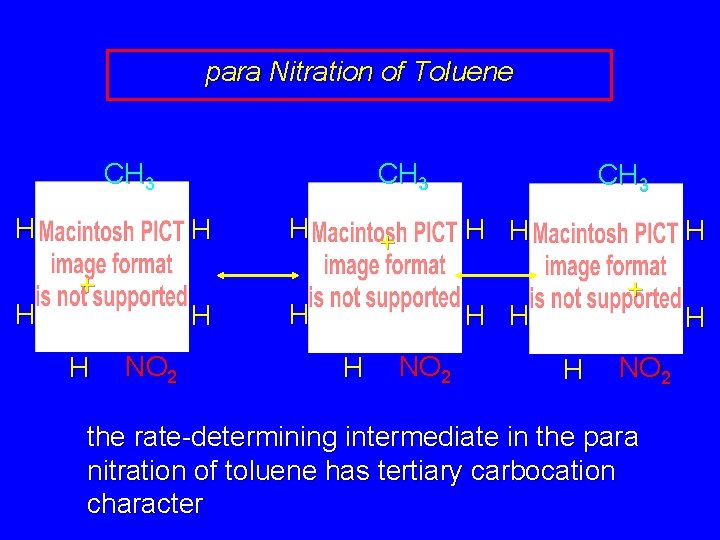
para Nitration of Toluene CH 3 H H CH 3 H + H H NO 2 H CH 3 H H + H H H NO 2 the rate-determining intermediate in the para nitration of toluene has tertiary carbocation character H

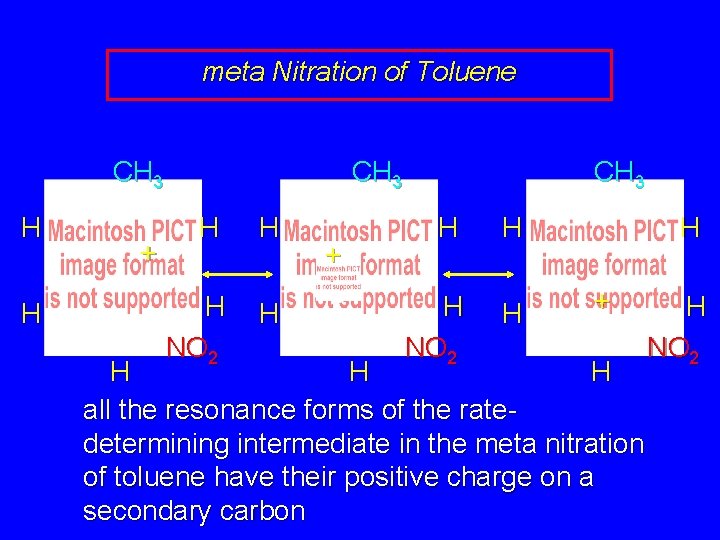
meta Nitration of Toluene CH 3 H + H H NO 2

meta Nitration of Toluene CH 3 H + H H CH 3 H H H NO 2 H H + H H NO 2

meta Nitration of Toluene CH 3 H H + CH 3 H H H NO 2 H H + H H H all the resonance forms of the ratedetermining intermediate in the meta nitration of toluene have their positive charge on a secondary carbon H NO 2

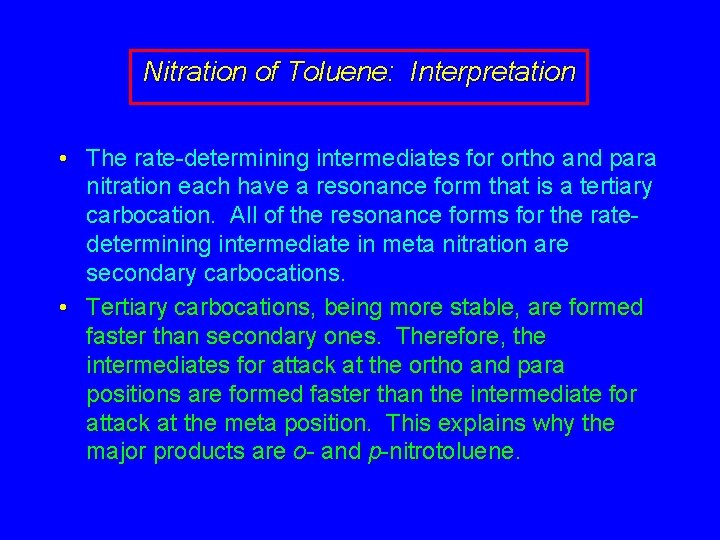
Nitration of Toluene: Interpretation • The rate-determining intermediates for ortho and para nitration each have a resonance form that is a tertiary carbocation. All of the resonance forms for the ratedetermining intermediate in meta nitration are secondary carbocations. • Tertiary carbocations, being more stable, are formed faster than secondary ones. Therefore, the intermediates for attack at the ortho and para positions are formed faster than the intermediate for attack at the meta position. This explains why the major products are o- and p-nitrotoluene.

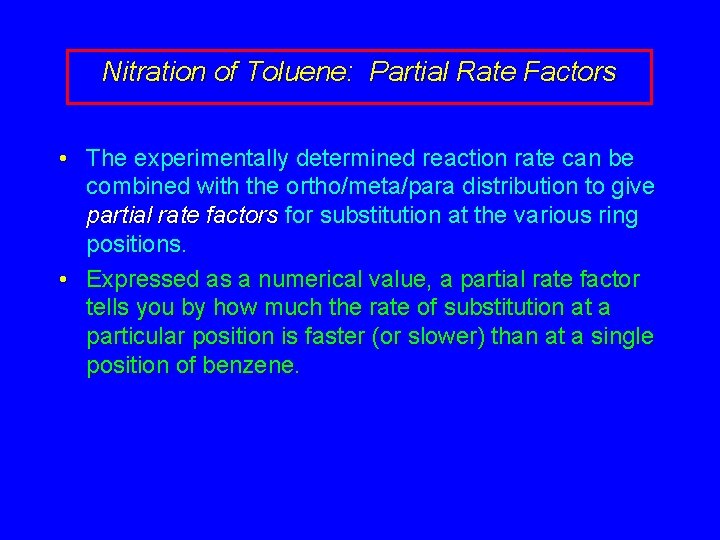
Nitration of Toluene: Partial Rate Factors • The experimentally determined reaction rate can be combined with the ortho/meta/para distribution to give partial rate factors for substitution at the various ring positions. • Expressed as a numerical value, a partial rate factor tells you by how much the rate of substitution at a particular position is faster (or slower) than at a single position of benzene.

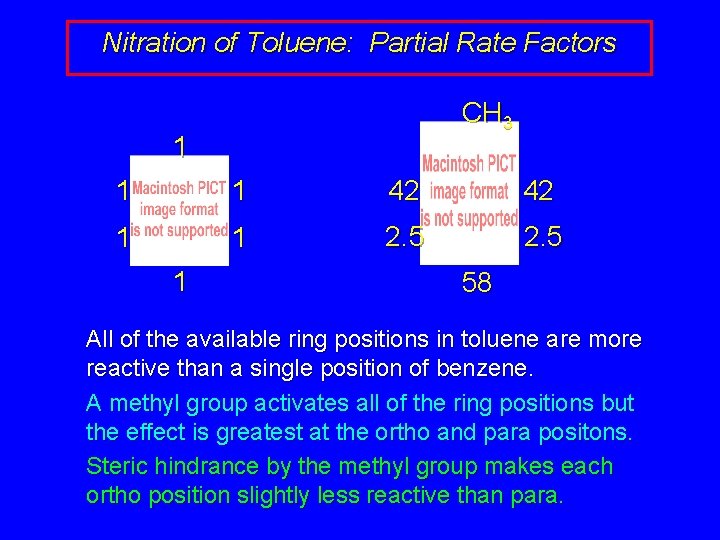
Nitration of Toluene: Partial Rate Factors CH 3 1 1 1 42 42 1 1 2. 5 1 58 All of the available ring positions in toluene are more reactive than a single position of benzene. A methyl group activates all of the ring positions but the effect is greatest at the ortho and para positons. Steric hindrance by the methyl group makes each ortho position slightly less reactive than para.

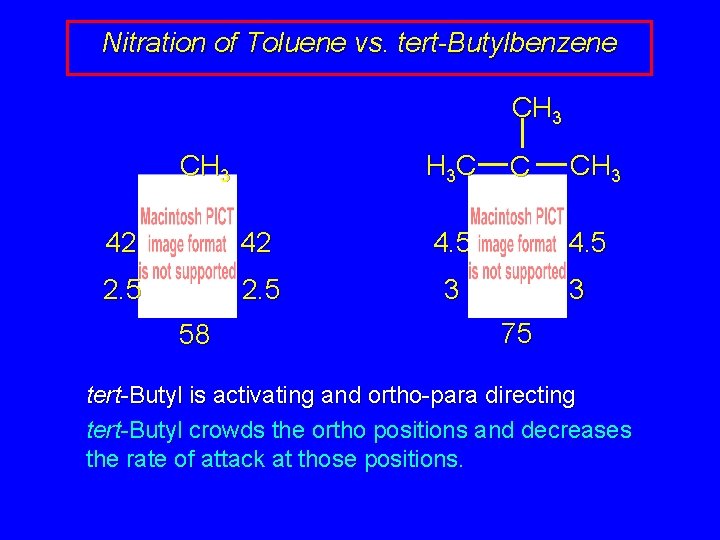
Nitration of Toluene vs. tert-Butylbenzene CH 3 H 3 C 42 42 4. 5 2. 5 3 58 C CH 3 4. 5 3 75 tert-Butyl is activating and ortho-para directing tert-Butyl crowds the ortho positions and decreases the rate of attack at those positions.

Generalization all alkyl groups are activating and ortho-para directing

12. 11 Rate and Regioselectivity in the Nitration of (Trifluoromethyl)benzene

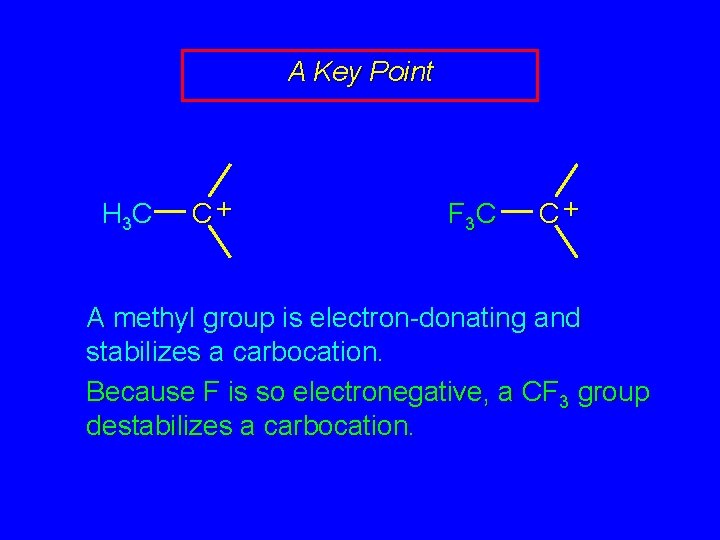
A Key Point H 3 C C+ F 3 C C+ A methyl group is electron-donating and stabilizes a carbocation. Because F is so electronegative, a CF 3 group destabilizes a carbocation.

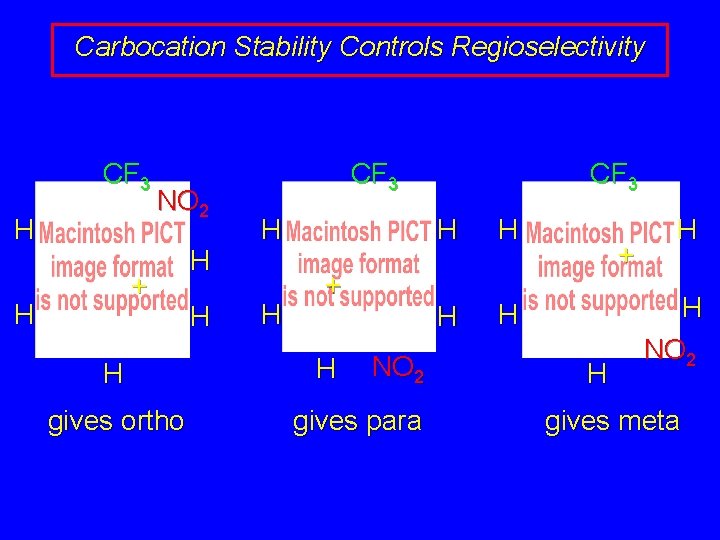
Carbocation Stability Controls Regioselectivity CF 3 H NO 2 + H H gives ortho H H CF 3 H H + H NO 2 gives para CF 3 H H + H H H NO 2 gives meta

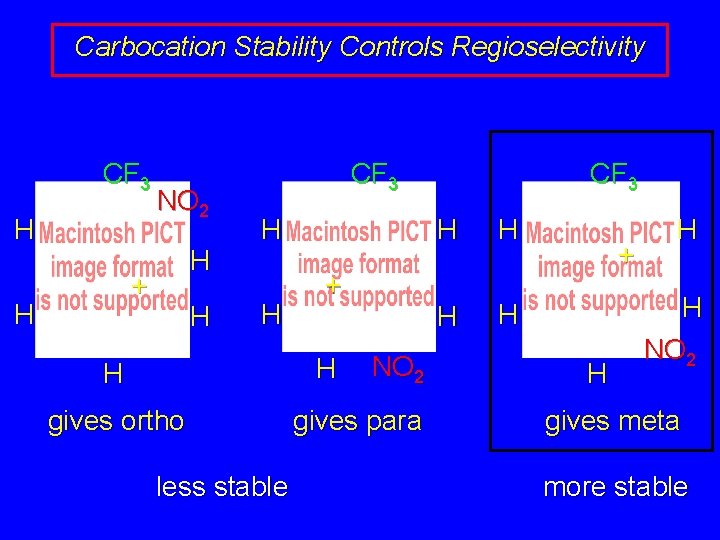
Carbocation Stability Controls Regioselectivity CF 3 H NO 2 H + H H CF 3 H H + H H gives ortho less stable NO 2 gives para CF 3 H H + H H H NO 2 gives meta more stable

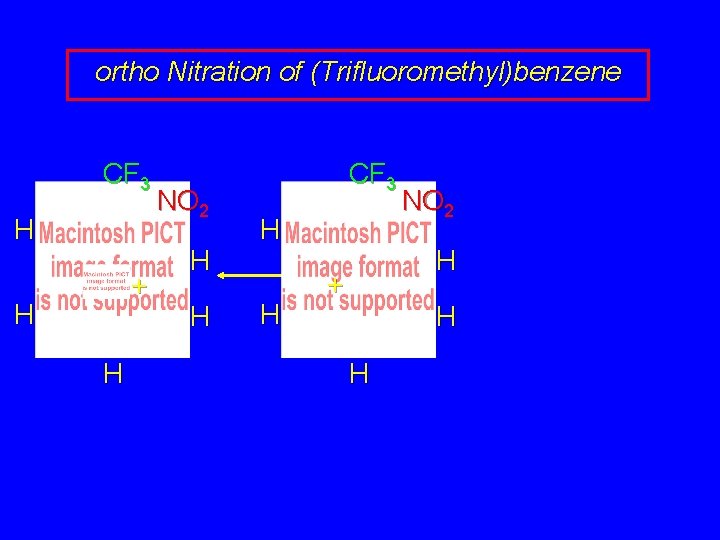
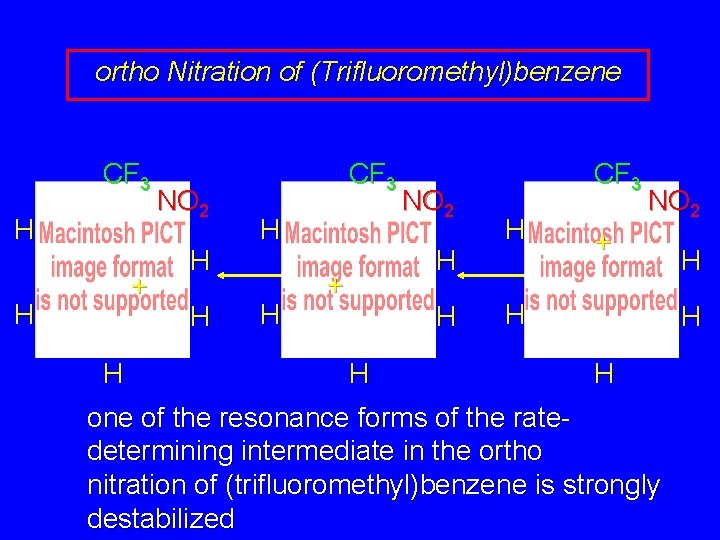
ortho Nitration of (Trifluoromethyl)benzene CF 3 H + H H NO 2 H H

ortho Nitration of (Trifluoromethyl)benzene CF 3 H + H H NO 2 H H CF 3 H H NO 2 H + H H

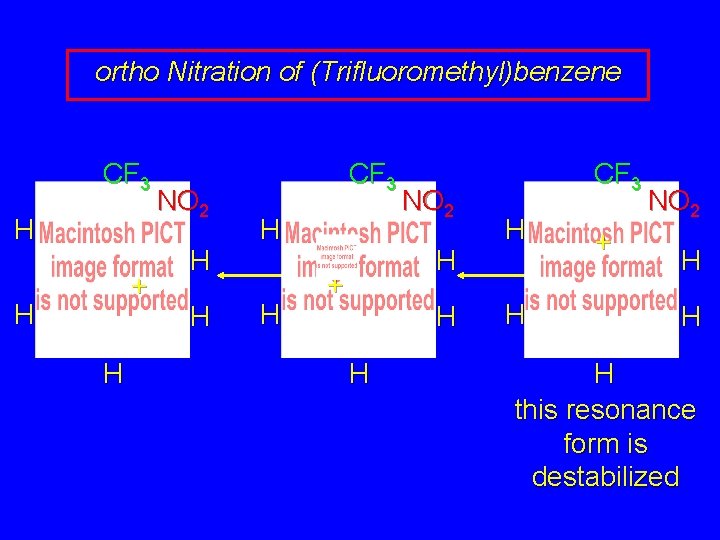
ortho Nitration of (Trifluoromethyl)benzene CF 3 H + H H NO 2 H H CF 3 H H NO 2 H + H H CF 3 H H + NO 2 H H H this resonance form is destabilized

ortho Nitration of (Trifluoromethyl)benzene CF 3 H + H H NO 2 H H CF 3 H H NO 2 H + H H CF 3 H NO 2 + H H one of the resonance forms of the ratedetermining intermediate in the ortho nitration of (trifluoromethyl)benzene is strongly destabilized

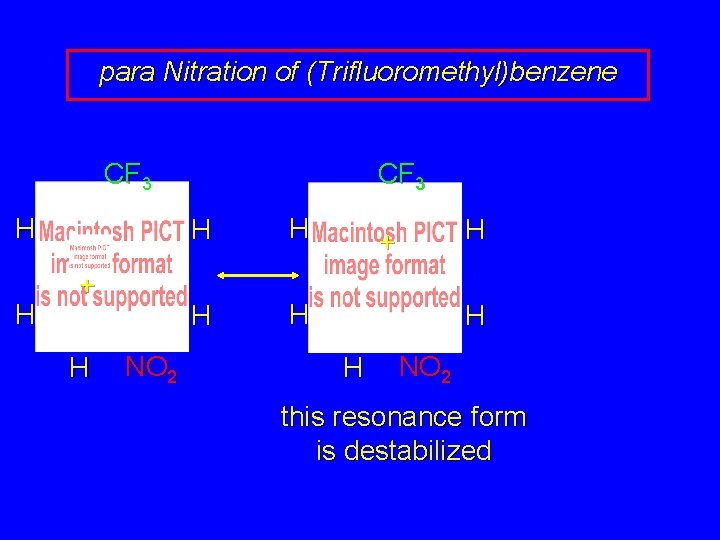
para Nitration of (Trifluoromethyl)benzene CF 3 H H H + H H NO 2

para Nitration of (Trifluoromethyl)benzene CF 3 H H + H NO 2 CF 3 H H H + H H NO 2 this resonance form is destabilized

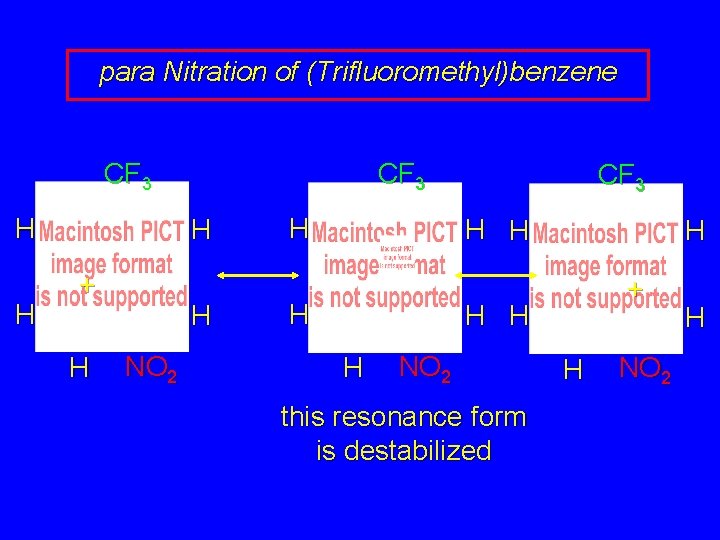
para Nitration of (Trifluoromethyl)benzene CF 3 H H CF 3 H + H H NO 2 H CF 3 H H + H H H NO 2 this resonance form is destabilized H NO 2 H

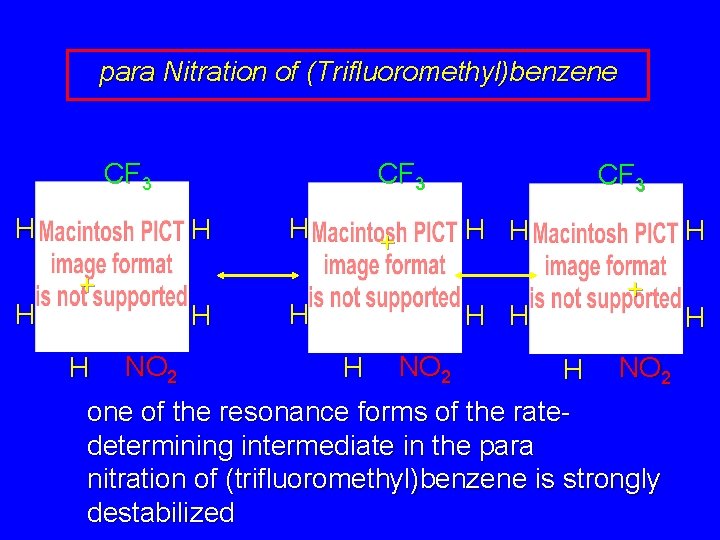
para Nitration of (Trifluoromethyl)benzene CF 3 H H CF 3 H + H H NO 2 H CF 3 H H + H H NO 2 H + H NO 2 one of the resonance forms of the ratedetermining intermediate in the para nitration of (trifluoromethyl)benzene is strongly destabilized H

meta Nitration of (Trifluoromethyl)benzene CF 3 H + H H NO 2

meta Nitration of (Trifluoromethyl)benzene CF 3 H + H H CF 3 H H H NO 2 H H + H H NO 2

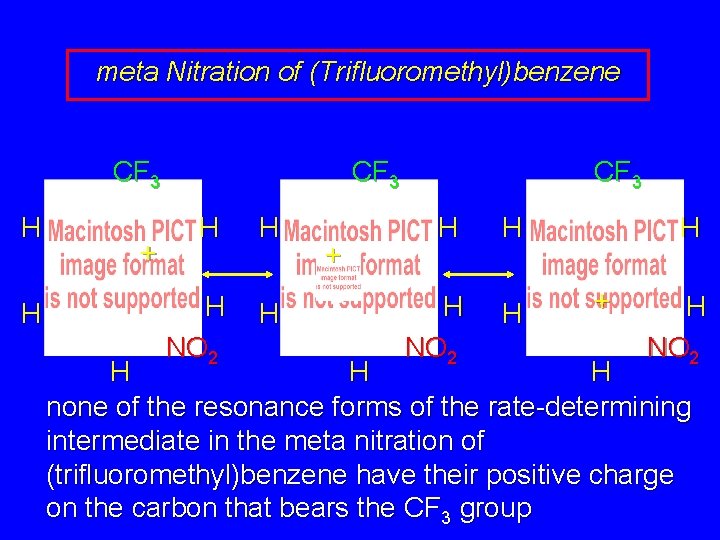
meta Nitration of (Trifluoromethyl)benzene CF 3 H H + CF 3 H H H NO 2 H H + H NO 2 H H H none of the resonance forms of the rate-determining intermediate in the meta nitration of (trifluoromethyl)benzene have their positive charge on the carbon that bears the CF 3 group

Nitration of (Trifluoromethyl)benzene: Interpretation The rate-determining intermediates for ortho and para nitration each have a resonance form in which the positive charge is on a carbon that bears a CF 3 group. Such a resonance structure is strongly destabilized. The intermediate in meta nitration avoids such a structure. It is the least unstable of three unstable intermediates and is the one from which most of the product is formed.

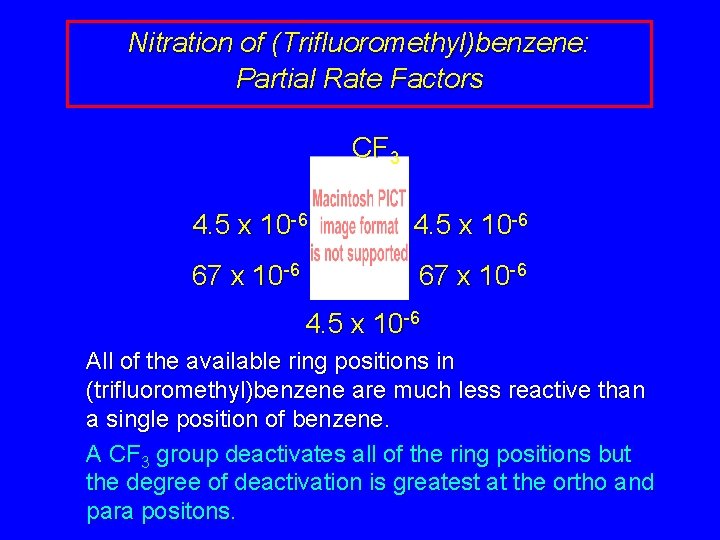
Nitration of (Trifluoromethyl)benzene: Partial Rate Factors CF 3 4. 5 x 10 -6 67 x 10 -6 4. 5 x 10 -6 All of the available ring positions in (trifluoromethyl)benzene are much less reactive than a single position of benzene. A CF 3 group deactivates all of the ring positions but the degree of deactivation is greatest at the ortho and para positons.
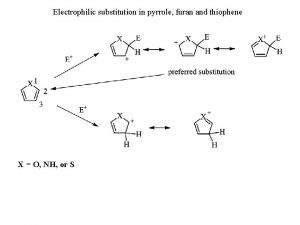
 Thiophene nucleophilic substitution
Thiophene nucleophilic substitution What is
What is Electrophilic aromatic substitution
Electrophilic aromatic substitution Toluene electrophilic aromatic substitution
Toluene electrophilic aromatic substitution Difference between electrophilic and nucleophilic
Difference between electrophilic and nucleophilic Carbonyl group electrophile
Carbonyl group electrophile Contoh senyawa heterosiklik
Contoh senyawa heterosiklik Medicinal uses of quinoline
Medicinal uses of quinoline Electrophilic substitution reaction of isoquinoline
Electrophilic substitution reaction of isoquinoline Electrophilic addition hbr
Electrophilic addition hbr Aliphatic amines
Aliphatic amines Basicity of amines
Basicity of amines Classification of amines
Classification of amines Alkane functional group
Alkane functional group Define medicinal plant
Define medicinal plant Aromatic himalayan plant
Aromatic himalayan plant Aniline ir spectrum
Aniline ir spectrum Is pyridine aromatic
Is pyridine aromatic Nitro group ir peak
Nitro group ir peak Cycloheptatrienyl cation
Cycloheptatrienyl cation Examples of non aromatic compounds
Examples of non aromatic compounds What is n in huckel rule
What is n in huckel rule Aromaticity in benzenoid and non benzenoid compounds
Aromaticity in benzenoid and non benzenoid compounds Annulene is aromatic or not
Annulene is aromatic or not Aromatic compounds huckel rule
Aromatic compounds huckel rule Which of the following molecules are aromatic hydrocarbons
Which of the following molecules are aromatic hydrocarbons Non aromatic compounds
Non aromatic compounds Aromatic amino acids
Aromatic amino acids Quaternary amine
Quaternary amine Do aromatic amines give hinsberg test
Do aromatic amines give hinsberg test Aliphatic vs aromatic
Aliphatic vs aromatic Aromatic compounds
Aromatic compounds Slidetodoc.com
Slidetodoc.com Aromatic compounds undergo
Aromatic compounds undergo Unsaturated aromatic compounds
Unsaturated aromatic compounds Cinnamon aromatic water
Cinnamon aromatic water Cyclopentadienone antiaromatic
Cyclopentadienone antiaromatic Activators and deactivators chart
Activators and deactivators chart Cycloheptatriene cation is aromatic or not
Cycloheptatriene cation is aromatic or not Compound orange spirit
Compound orange spirit Aromatic amino acids
Aromatic amino acids Electron withdrawing groups
Electron withdrawing groups Caracter aromatic
Caracter aromatic Caracter aromatic
Caracter aromatic