Why should You learn Chemistry Required Pharmacists Doctors














- Slides: 49

Why should You learn Chemistry? • Required – – Pharmacists Doctors Dentists Chem Engineer, etc. • Need it to pass Standardized Exams, Boards, etc. – – MCAT PCAT DAT OAT, etc • Understanding of Nature – – Drug Design: Manipulate molecules for better medicine Better utilize Energy: Manipulate molecules for better fuels Better understand Life: Biochemistry/Molecular Biology Create better technology

Outline of presentation • What is Chemistry? • Analytical Chemistry • What is in a sample? • How much is present? • Biochemistry- study of life’s chemistry • Inorganic Chemistry- all other elements except carbon • Separation Science • Organic chemistry- study of carbon compounds • Physical Chemistry- study of chemical events based on natural physical phenom

Organic Chemistry is the study of compounds that consist of carbon atoms

What is so special about Carbon? • Interesting facts: – 46 million chemical compounds are known in nature. – 99% of them contain carbon (thus organic) – Why? ?

Biochemistry “What are the unique chemical foundations of all life and what is different”

Why should you Learn Biological Chemistry: Drug Design What knowledge is required for drug design? • Know structures of protein targets in the body • Biochemistry • Molecular Biology • Know how to manipulate organic molecules to create new compounds with specific properties Sildenafil • Organic chemistry • Chemical Synthesis

Analytical Chemistry: Science of Chemical Analysis

Careers in Chemistry • • Chemist/Biochemist Chemical Engineer Industrial Chemist Chemical Technician • B. S. (4 yr), M. S. (6 -7 yr), Ph. D. (8 -12 yr)

Matter and Measurement By Doba D. Jackson, Ph. D. Associate Professor of Chemistry & Biochemistry Huntingdon College

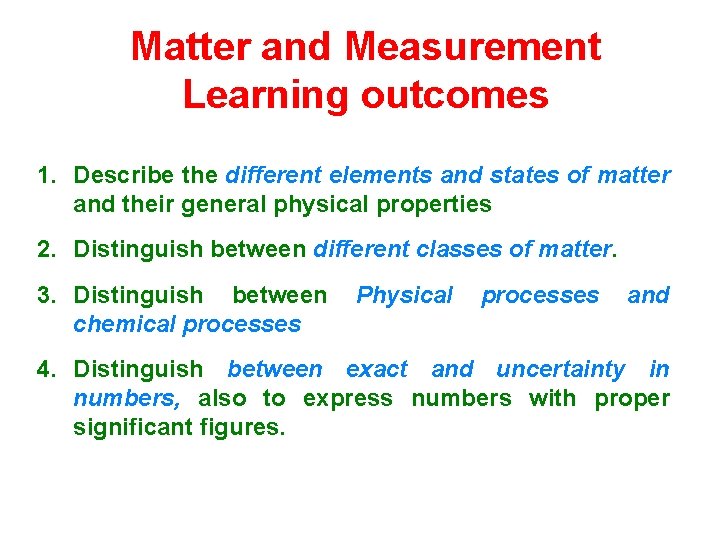
Matter and Measurement Learning outcomes 1. Describe the different elements and states of matter and their general physical properties 2. Distinguish between different classes of matter. 3. Distinguish between chemical processes Physical processes and 4. Distinguish between exact and uncertainty in numbers, also to express numbers with proper significant figures.

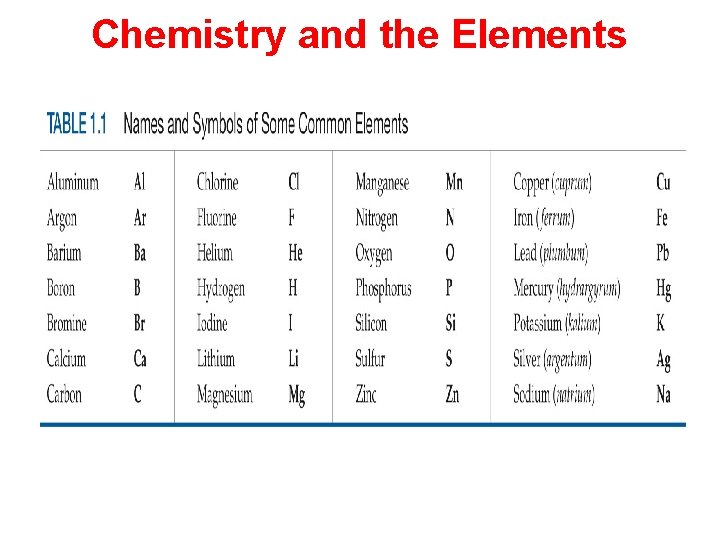
Chemistry and the Elements


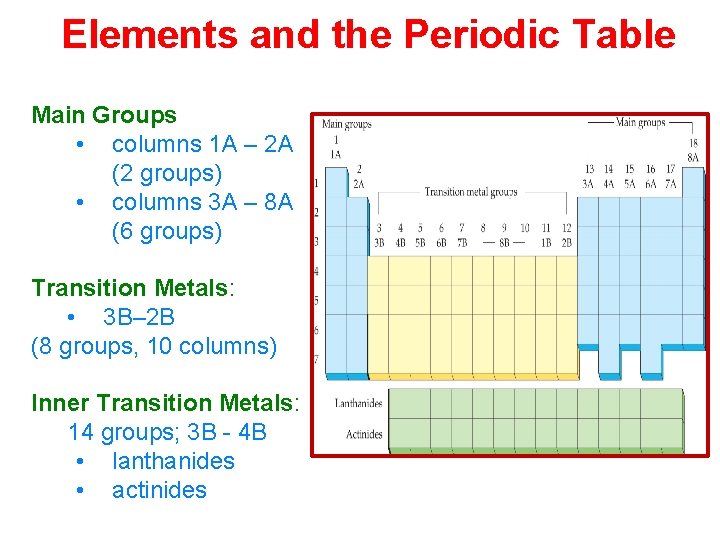
Elements and the Periodic Table Main Groups • columns 1 A – 2 A (2 groups) • columns 3 A – 8 A (6 groups) Transition Metals: • 3 B– 2 B (8 groups, 10 columns) Inner Transition Metals: 14 groups; 3 B - 4 B • lanthanides • actinides

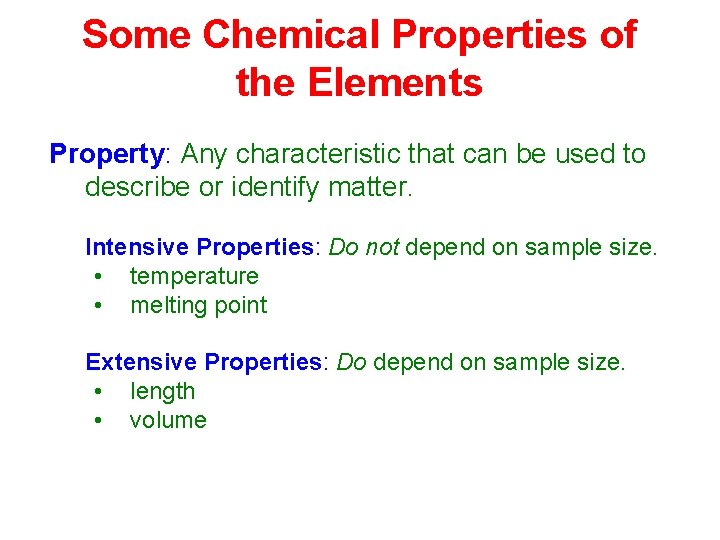
Some Chemical Properties of the Elements Property: Any characteristic that can be used to describe or identify matter. Intensive Properties: Do not depend on sample size. • temperature • melting point Extensive Properties: Do depend on sample size. • length • volume

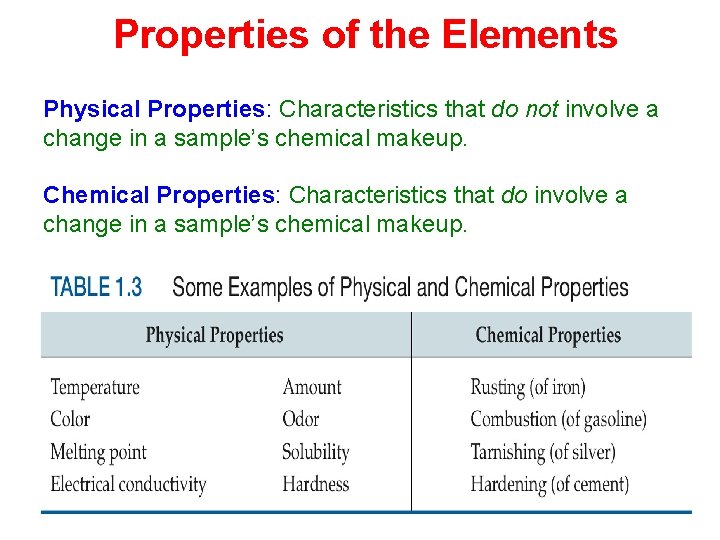
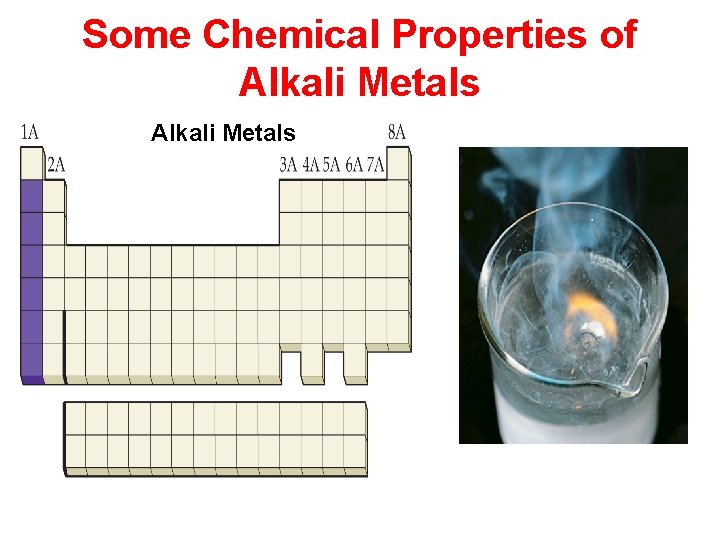
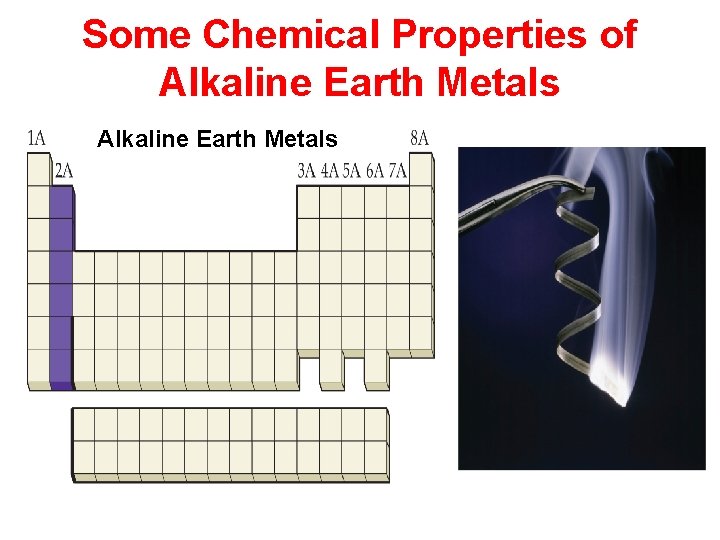
Properties of the Elements Physical Properties: Characteristics that do not involve a change in a sample’s chemical makeup. Chemical Properties: Characteristics that do involve a change in a sample’s chemical makeup.

Some Chemical Properties of Alkali Metals

Some Chemical Properties of Alkaline Earth Metals

Some Chemical Properties of the Halogens

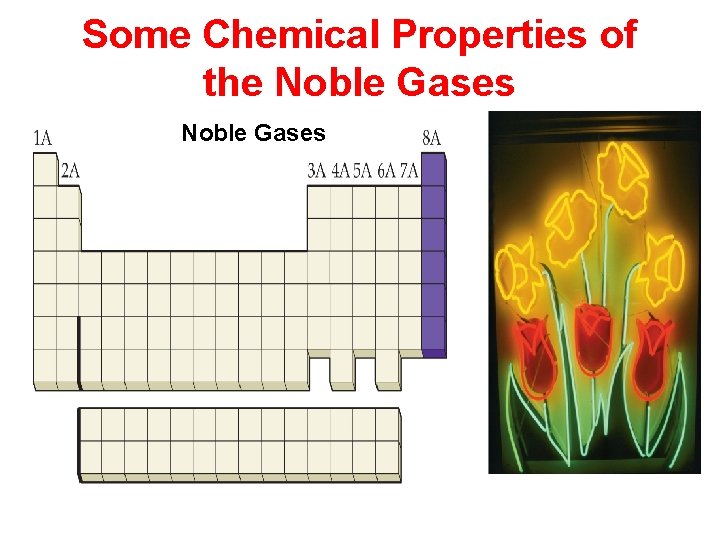
Some Chemical Properties of the Noble Gases

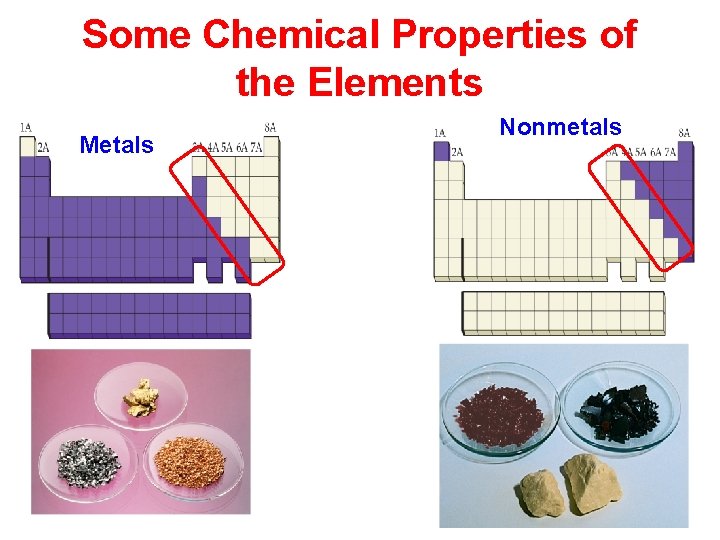
Some Chemical Properties of the Elements Metals Nonmetals

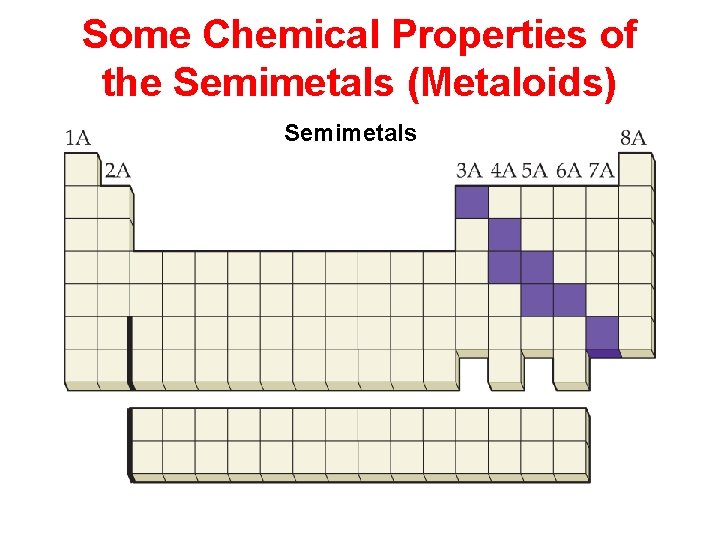
Some Chemical Properties of the Semimetals (Metaloids) Semimetals

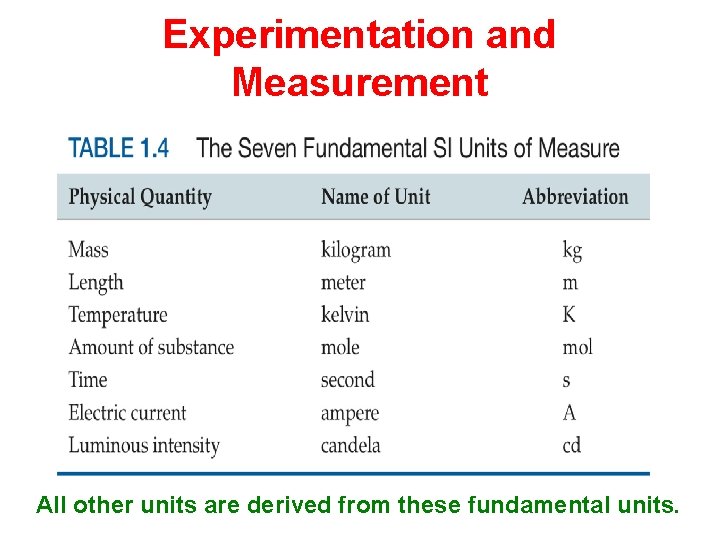
Experimentation and Measurement All other units are derived from these fundamental units.


Mass and its Measurement Mass: Amount of matter in an object. Matter: Describes anything with a physical presence—anything you can touch, taste, or smell. Weight: Measures the force with which gravity pulls on an object.

Mass and Its Measurement

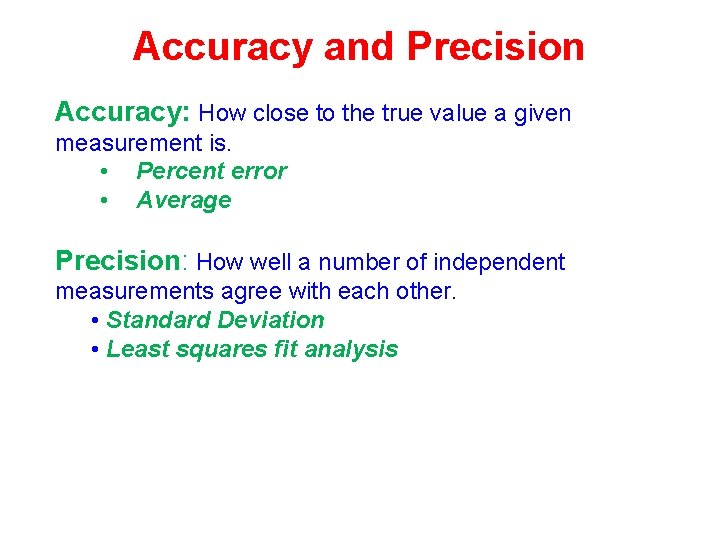
Accuracy and Precision Accuracy: How close to the true value a given measurement is. • Percent error • Average Precision: How well a number of independent measurements agree with each other. • Standard Deviation • Least squares fit analysis

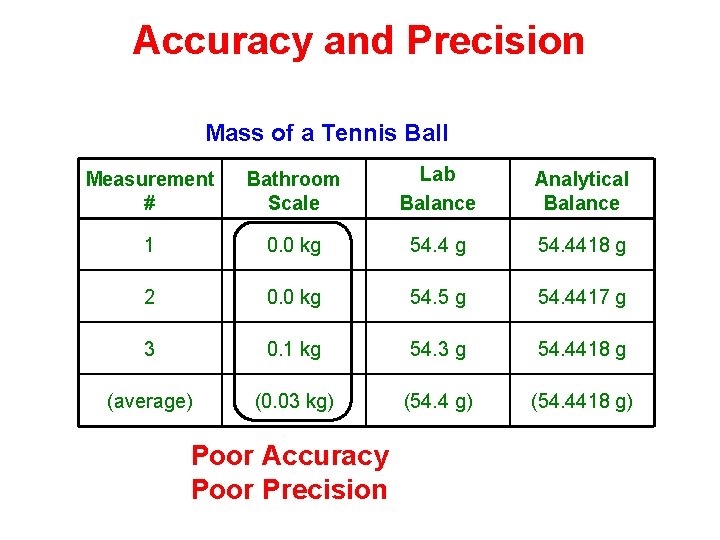
Accuracy and Precision Mass of a Tennis Ball Measurement # Bathroom Scale Lab Balance Analytical Balance 1 0. 0 kg 54. 4418 g 2 0. 0 kg 54. 5 g 54. 4417 g 3 0. 1 kg 54. 3 g 54. 4418 g (average) (0. 03 kg) (54. 4418 g) Poor Accuracy Poor Precision

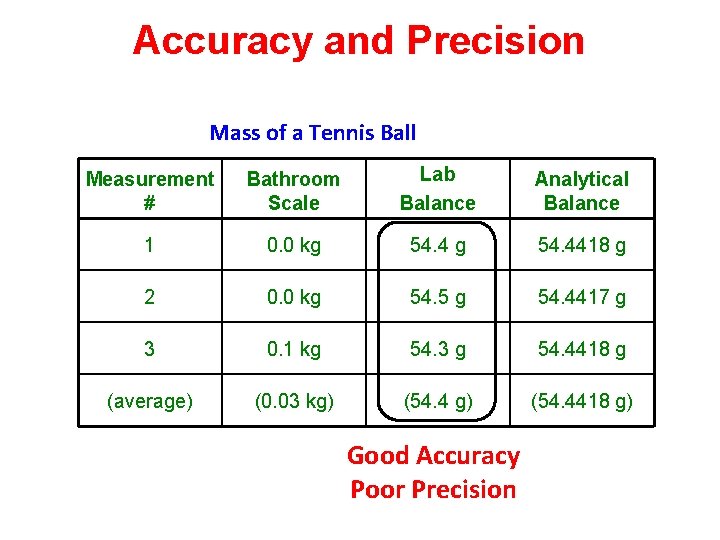
Accuracy and Precision Mass of a Tennis Ball Measurement # Bathroom Scale Lab Balance Analytical Balance 1 0. 0 kg 54. 4418 g 2 0. 0 kg 54. 5 g 54. 4417 g 3 0. 1 kg 54. 3 g 54. 4418 g (average) (0. 03 kg) (54. 4418 g) Good Accuracy Poor Precision

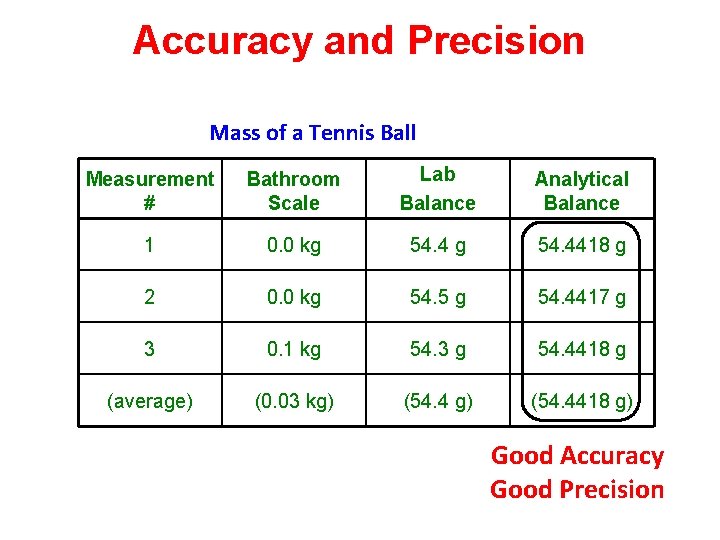
Accuracy and Precision Mass of a Tennis Ball Measurement # Bathroom Scale Lab Balance Analytical Balance 1 0. 0 kg 54. 4418 g 2 0. 0 kg 54. 5 g 54. 4417 g 3 0. 1 kg 54. 3 g 54. 4418 g (average) (0. 03 kg) (54. 4418 g) Good Accuracy Good Precision

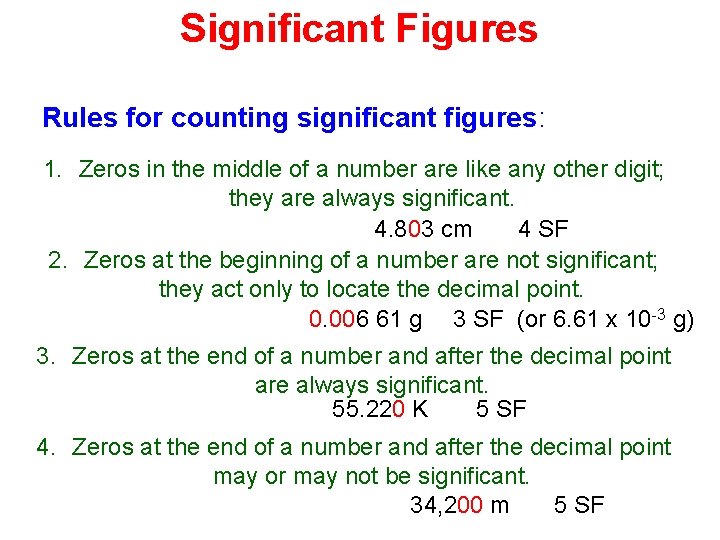
Significant Figures Rules for counting significant figures: 1. Zeros in the middle of a number are like any other digit; they are always significant. 4. 803 cm 4 SF 2. Zeros at the beginning of a number are not significant; they act only to locate the decimal point. 0. 006 61 g 3 SF (or 6. 61 x 10 -3 g) 3. Zeros at the end of a number and after the decimal point are always significant. 55. 220 K 5 SF 4. Zeros at the end of a number and after the decimal point may or may not be significant. 34, 200 m 5 SF

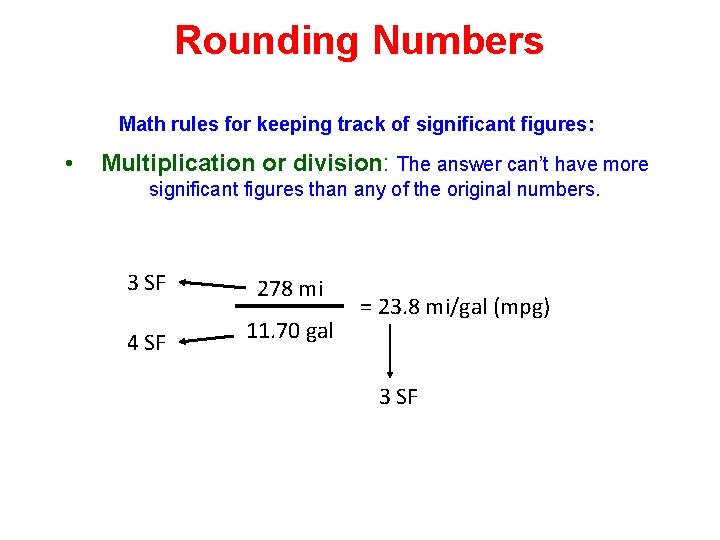
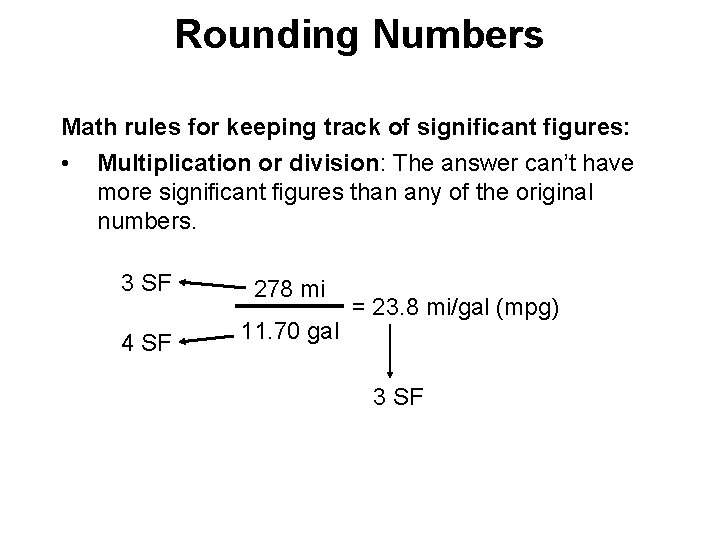
Rounding Numbers Math rules for keeping track of significant figures: • Multiplication or division: The answer can’t have more significant figures than any of the original numbers. 3 SF 278 mi 4 SF 11. 70 gal = 23. 8 mi/gal (mpg) 3 SF

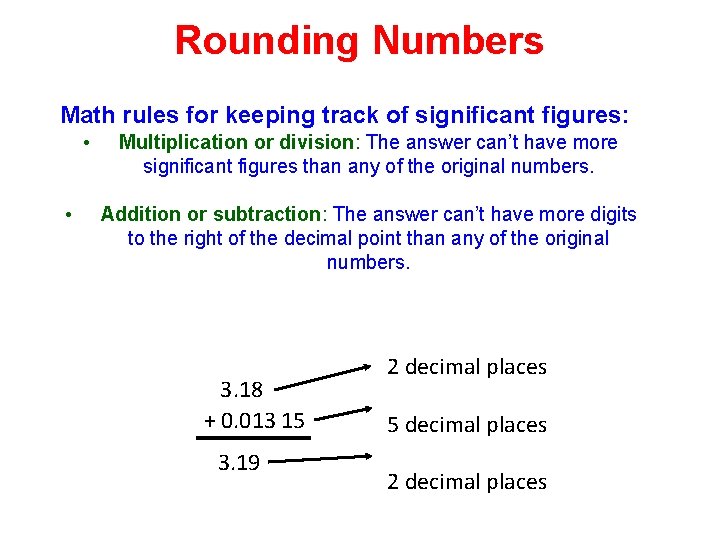
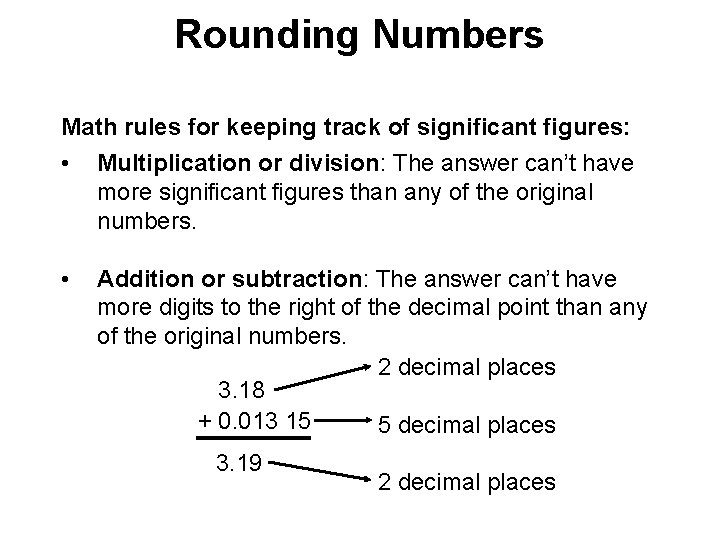
Rounding Numbers Math rules for keeping track of significant figures: • • Multiplication or division: The answer can’t have more significant figures than any of the original numbers. Addition or subtraction: The answer can’t have more digits to the right of the decimal point than any of the original numbers. 3. 18 + 0. 013 15 3. 19 2 decimal places 5 decimal places 2 decimal places

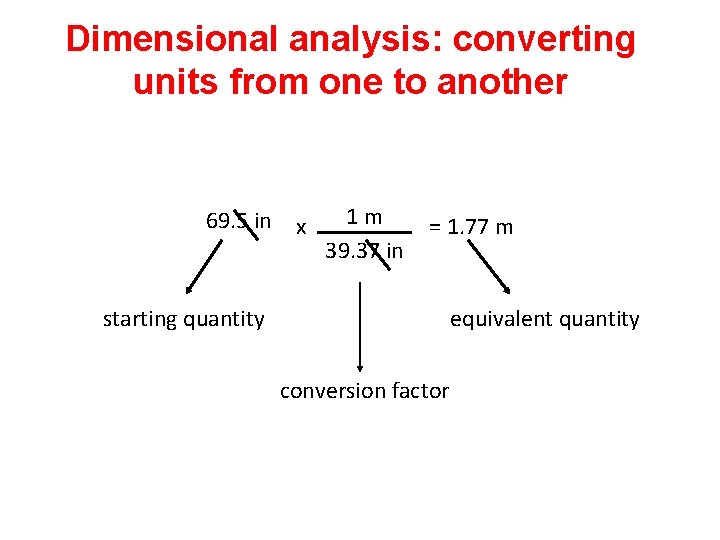
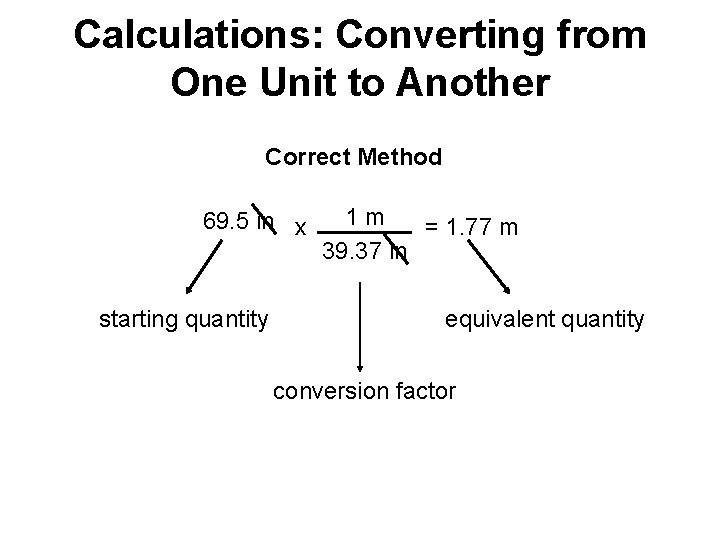
Dimensional analysis: converting units from one to another 69. 5 in x 1 m 39. 37 in = 1. 77 m starting quantity equivalent quantity conversion factor

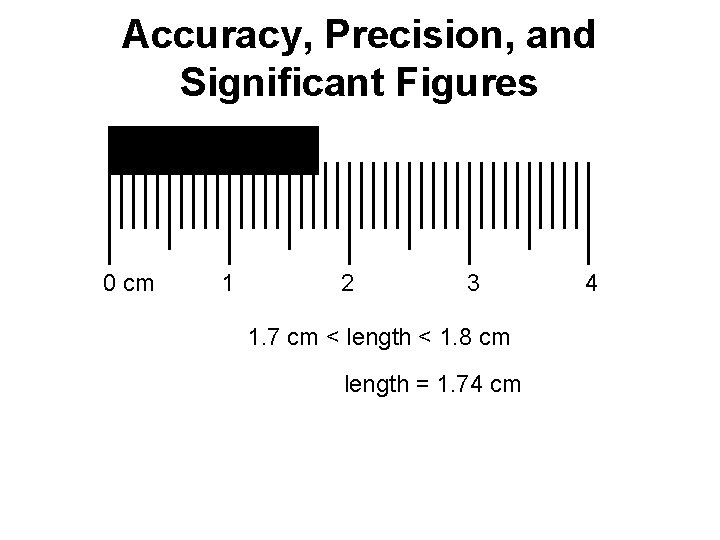
Accuracy, Precision, and Significant Figures Significant figures: The total number of digits recorded in a measured or calculated quantity. They come from uncertainty in any measurement. Generally the last digit in a reported measurement is uncertain (estimated). Exact numbers and relationships (7 days in a week, 30 students in a class, etc. ) effectively have an infinite number of significant figures.

Accuracy, Precision, and Significant Figures 0 cm 1 2 3 1. 7 cm < length < 1. 8 cm length = 1. 74 cm 4

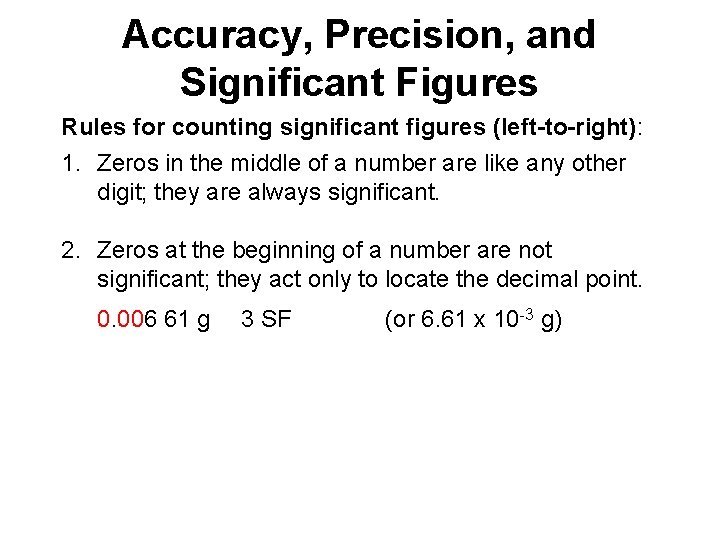
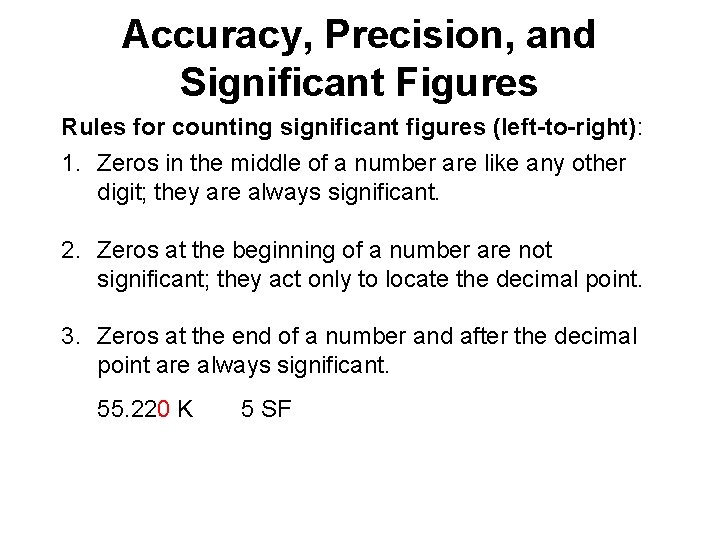
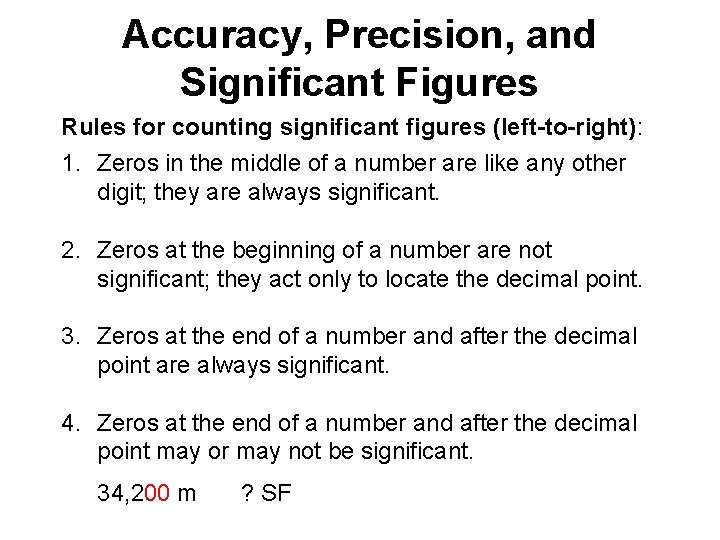
Accuracy, Precision, and Significant Figures Rules for counting significant figures (left-to-right): 1. Zeros in the middle of a number are like any other digit; they are always significant. 4. 803 cm 4 SF

Accuracy, Precision, and Significant Figures Rules for counting significant figures (left-to-right): 1. Zeros in the middle of a number are like any other digit; they are always significant. 2. Zeros at the beginning of a number are not significant; they act only to locate the decimal point. 0. 006 61 g 3 SF (or 6. 61 x 10 -3 g)

Accuracy, Precision, and Significant Figures Rules for counting significant figures (left-to-right): 1. Zeros in the middle of a number are like any other digit; they are always significant. 2. Zeros at the beginning of a number are not significant; they act only to locate the decimal point. 3. Zeros at the end of a number and after the decimal point are always significant. 55. 220 K 5 SF

Accuracy, Precision, and Significant Figures Rules for counting significant figures (left-to-right): 1. Zeros in the middle of a number are like any other digit; they are always significant. 2. Zeros at the beginning of a number are not significant; they act only to locate the decimal point. 3. Zeros at the end of a number and after the decimal point are always significant. 4. Zeros at the end of a number and after the decimal point may or may not be significant. 34, 200 m ? SF

Rounding Numbers Math rules for keeping track of significant figures: • Multiplication or division: The answer can’t have more significant figures than any of the original numbers. 3 SF 278 mi 4 SF 11. 70 gal = 23. 8 mi/gal (mpg) 3 SF

Rounding Numbers Math rules for keeping track of significant figures: • Multiplication or division: The answer can’t have more significant figures than any of the original numbers. • Addition or subtraction: The answer can’t have more digits to the right of the decimal point than any of the original numbers. 2 decimal places 3. 18 + 0. 013 15 5 decimal places 3. 19 2 decimal places

Rounding Numbers Rules for rounding off numbers: 1. If the first digit you remove is less than 5, round down by dropping it and all following digits. 5. 664 525 = 5. 66

Rounding Numbers Rules for rounding off numbers: 1. If the first digit you remove is less than 5, round down by dropping it and all following digits. 2. If the first digit you remove is 6 or greater, round up by adding 1 to the digit on the left. 5. 664 525 = 5. 7

Rounding Numbers Rules for rounding off numbers: 1. If the first digit you remove is less than 5, round down by dropping it and all following digits. 2. If the first digit you remove is 6 or greater, round up by adding 1 to the digit on the left. 3. If the first digit you remove is 5 and there are more nonzero digits following, round up. 5. 664 525 = 5. 665

Rounding Numbers Rules for rounding off numbers: 1. If the first digit you remove is less than 5, round down by dropping it and all following digits. 2. If the first digit you remove is 6 or greater, round up by adding 1 to the digit on the left. 3. If the first digit you remove is 5 and there are more nonzero digits following, round up. 4. If the digit you remove is a 5 with nothing following, round down. 5. 664 525 = 5. 664 52

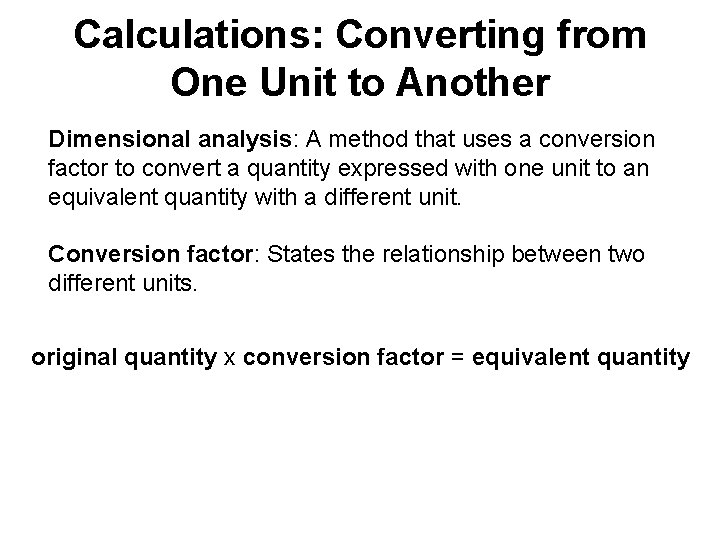
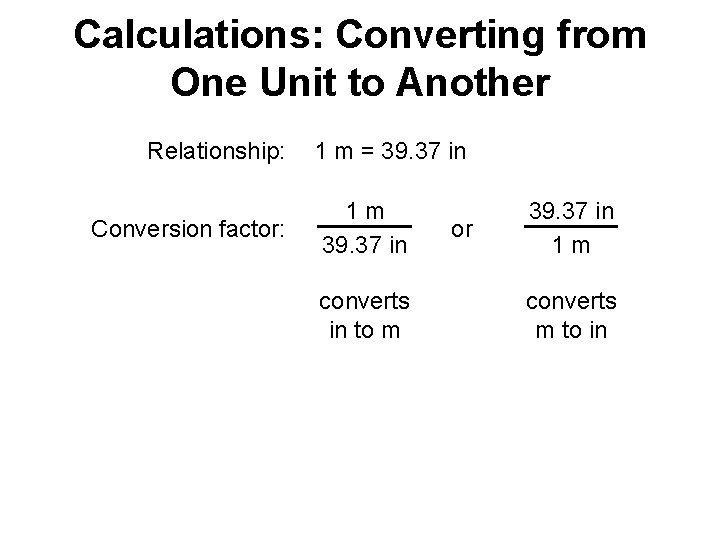
Calculations: Converting from One Unit to Another Dimensional analysis: A method that uses a conversion factor to convert a quantity expressed with one unit to an equivalent quantity with a different unit. Conversion factor: States the relationship between two different units. original quantity x conversion factor = equivalent quantity

Calculations: Converting from One Unit to Another Relationship: Conversion factor: 1 m = 39. 37 in 1 m 39. 37 in converts in to m or 39. 37 in 1 m converts m to in

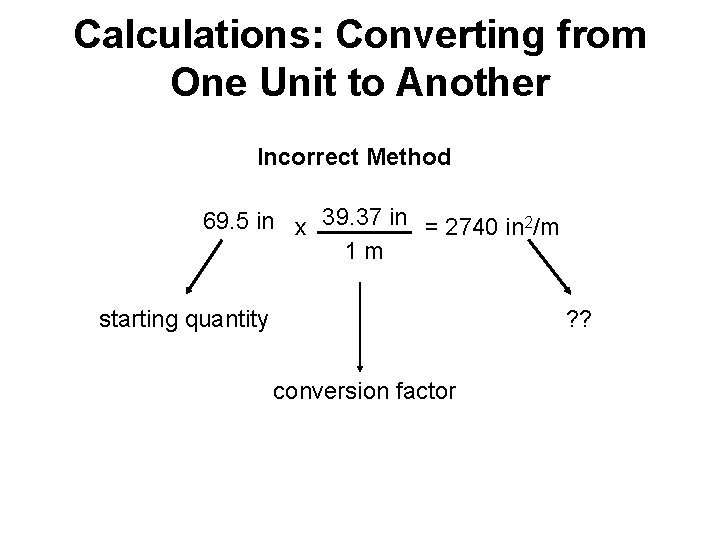
Calculations: Converting from One Unit to Another Incorrect Method 69. 5 in x 39. 37 in = 2740 in 2/m 1 m starting quantity ? ? conversion factor

Calculations: Converting from One Unit to Another Correct Method 69. 5 in x starting quantity 1 m = 1. 77 m 39. 37 in equivalent quantity conversion factor