Gentamicin A presentation for junior doctors and pharmacists






























- Slides: 44

Gentamicin A presentation for junior doctors and pharmacists

About this presentation • This e-Learning package is in two parts. The first part will give you some background on gentamicin and the dosing regimens used to ensure safe and effective therapy. The second part looks at the version of the Hartford extendedinterval regimen adopted by many trusts across Yorkshire. Author This e-Learning package was written by Kevin Frost, Lead Pharmacist for Antibiotics at Airedale NHS Foundation Trust It has been reviewed by Philip Howard, Consultant Pharmacist in Antibiotics at Leeds Teaching NHS Trust

Learning Objectives • After viewing this presentation, you should Understand the mechanism of action, indications and contra-indications of gentamicin Understand basic pharmacokinetics, as they apply to Gentamicin Be able to apply the above to use the Yorkshire Hartford Gentamicin regimen. Understand the principles of pharmacokinetic and synergistic gentamicin regimens.

Pharmacology • Gentamicin is a member of the aminoglycoside family of antibiotics. • Aminoglycosides are bactericidal antibiotics active against many aerobic gram-negative bacteria and some aerobic gram-positive bacteria. • Aminoglycosides act by disrupting protein synthesis at the bacterial ribosome.

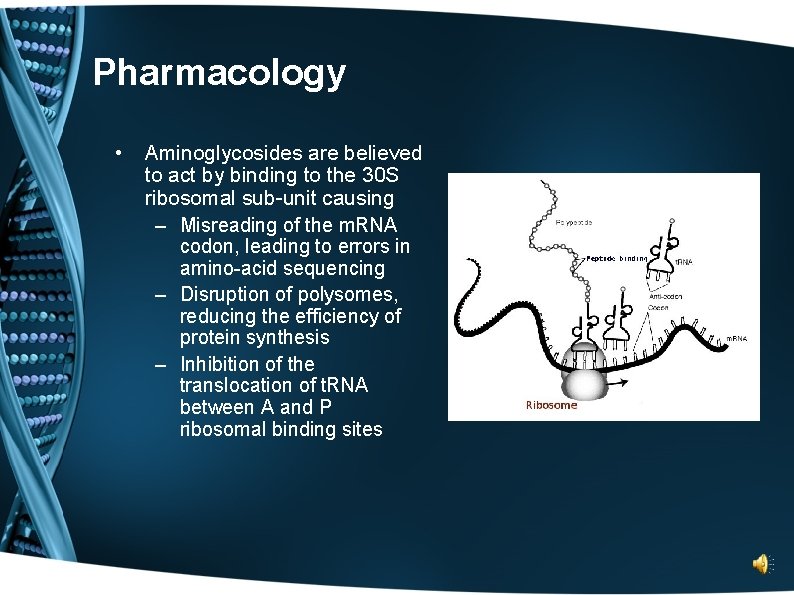
Pharmacology • Aminoglycosides are believed to act by binding to the 30 S ribosomal sub-unit causing – Misreading of the m. RNA codon, leading to errors in amino-acid sequencing – Disruption of polysomes, reducing the efficiency of protein synthesis – Inhibition of the translocation of t. RNA between A and P ribosomal binding sites

Side-effects of Gentamicin • Damage to the cochlear and vestibular apparatus – loss of balance, tinnitus, loss of hearing. • May cause renal damage - risk of nephrotoxicity is increased with prolonged treatment. – Concurrent use of other nephrotoxic drugs may exacerbate renal damage. – Use with ototoxic diuretics, e. g. furosemide, may increase risk of ototoxicity and nephrotoxicity. • May cause allergic reactions, nausea, vomiting and rashes.

Cautions and Contra-indications • Gentamicin is contra-indicated in severe renal impairment and pregnancy. • It is contra-indicated in myasthenia gravis (due to its effects on nerve cells). • It may increase the activity of neuromuscular blocking agents (although rarely clinically significant).

So why use Gentamicin? • Because it is very effective when used correctly. • If levels are monitored appropriately, severe renal impairment and ototoxicity rarely occur. • Low incidence of provoking Clostridium difficile infections

Pharmacokinetics • Study of how drugs pass through the body: – Includes; absorption, distribution, metabolism and excretion.

Absorption • Gentamicin is not readily absorbed from GI tract, so is given via intravenous route (may also be given IM).

Distribution • Gentamicin is highly hydrophilic, i. e. not distributed into body fat and minimally distributed into tissue fluids. • When calculating an appropriate dose, consider using the patient's lean mass (mass without excess fat = ideal body weight). • Usually calculate dose using lower of actual or ideal body weight.

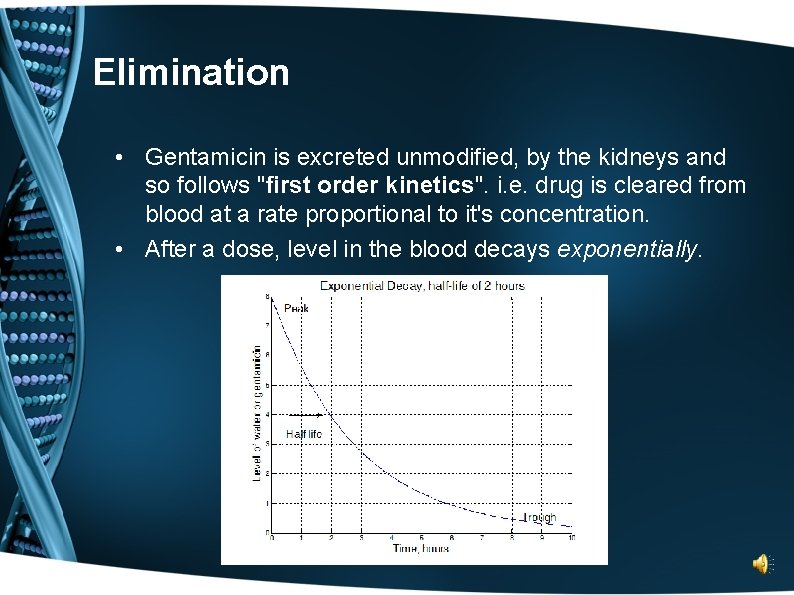
Elimination • Gentamicin is excreted unmodified, by the kidneys and so follows "first order kinetics". i. e. drug is cleared from blood at a rate proportional to it's concentration. • After a dose, level in the blood decays exponentially.

Elimination • Gentamicin is excreted unmodified, by the kidneys and so follows "first order kinetics". i. e. drug is cleared from blood at a rate proportional to it's concentration. • After a bolus dose, level in the blood decays exponentially. • Kidneys continuously removing a constant fraction of gentamicin in the blood. As level goes down with time, less is excreted. • If patient’s renal function compromised, excretion less efficient, so must be considered before commencing a course.

Dosing Regimens • Two types of regimens commonly used in UK: – Pharmacokinetic – Extended Interval. • A 1996 paper (1) looked at meta-analyses comparing these regimens and found: – Both regimens had equivalent efficacy, but; – Extended Interval gentamicin had reduced nephrotoxicity. • 1 Mega-analysis of meta-analysis: an examination of meta-analysis with an emphasis on once-daily aminoglycoside comparative trials. Pharmacotherapy. 1996 Nov-Dec; 16(6): 1093 -1102.

Extended Interval Dosing • May also be known as: – Once daily dosing/administration – Hartford dosing • Pioneered at Hartford hospital (Connecticut, USA) incorporating pharmacodynamic concepts of post-antibiotic effect and concentrationdependent kill. • Regimen maximises bacterial kill whilst minimising toxicity.

Hartford Regimen – key points • Hartford regimen is based on a consistent dose of 7 mg/kg gentamicin calculated from the lower of the ideal body weight or actual body weight. • Serum level is measured 6 -14 hours after first dose to determine dosage interval. • Nomogram is used to determine whether patient should receive gentamicin every 24 hours, 36 hours or 48 hours.

Patient suitability • Do NOT use Hartford regimen for – – Pregnant women Children < 16 years Urology surgery prophylaxis Any patient who has • Ascites • Cystic fibrosis • Endocarditis (unless requested by microbiology consultant) • Major burns • Renal transplant • Renal impairment – creatinine clearance <30 m. L/min Unless specifically recommended by microbiology

Calculating creatinine clearance • Don’t used automated e. GFR • Use a Cockcroft-Gault estimate: • Men: (140 – age) x Weight (kg) x 1. 23 Serum creatinine (micromol/L) • Women: (140 – age) x Weight x 1. 04 Serum creatinine

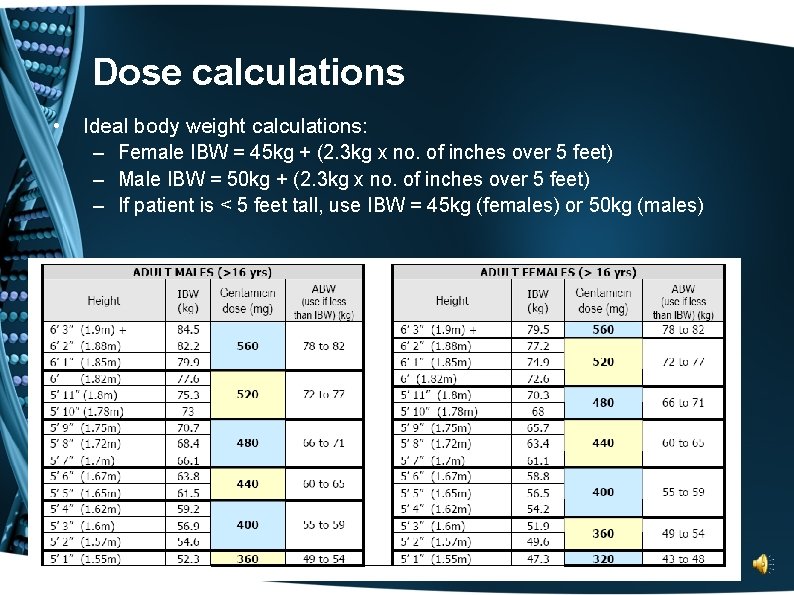
Dose calculations • Ideal body weight calculations: – Female IBW = 45 kg + (2. 3 kg x no. of inches over 5 feet) – Male IBW = 50 kg + (2. 3 kg x no. of inches over 5 feet) – If patient is < 5 feet tall, use IBW = 45 kg (females) or 50 kg (males)

Administration • Dilute gentamicin dose in 50 -100 m. L sodium chloride 0. 9% and give by intravenous infusion over 1 hour. • Record exact start time of the infusion on drug chart.

Measurement of levels • Look at your local policy for when your lab can receive gentamicin assays • Do not take blood sample from the IV line used for gentamicin administration! • Take one blood sample (ideally 10 m. L) between 6 and 14 hours after the start of first infusion in a plain tube (clotted blood). • Document on microbiology request form EXACT time and date infusion was set up and EXACT time and date sample was taken in addition to patient details and “Hartford Gentamicin Regimen. ”

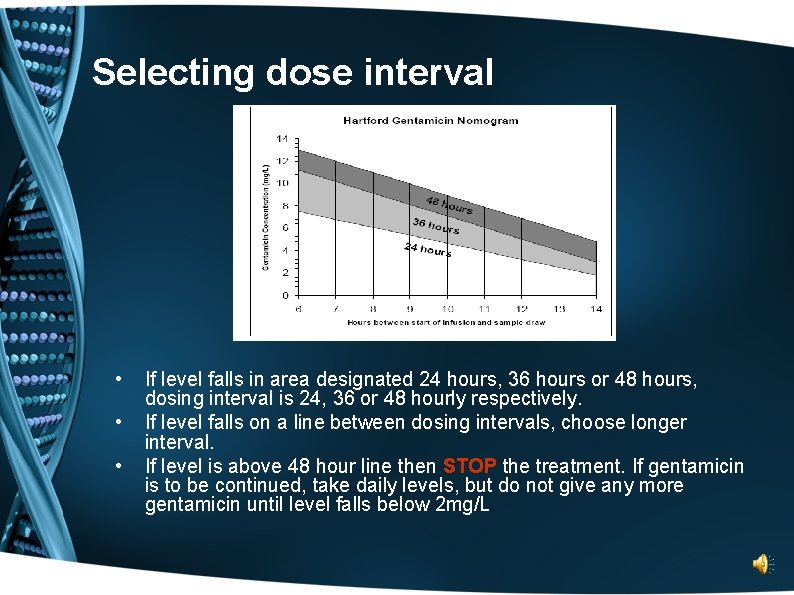
Selecting dose interval • • • If level falls in area designated 24 hours, 36 hours or 48 hours, dosing interval is 24, 36 or 48 hourly respectively. If level falls on a line between dosing intervals, choose longer interval. If level is above 48 hour line then STOP the treatment. If gentamicin is to be continued, take daily levels, but do not give any more gentamicin until level falls below 2 mg/L

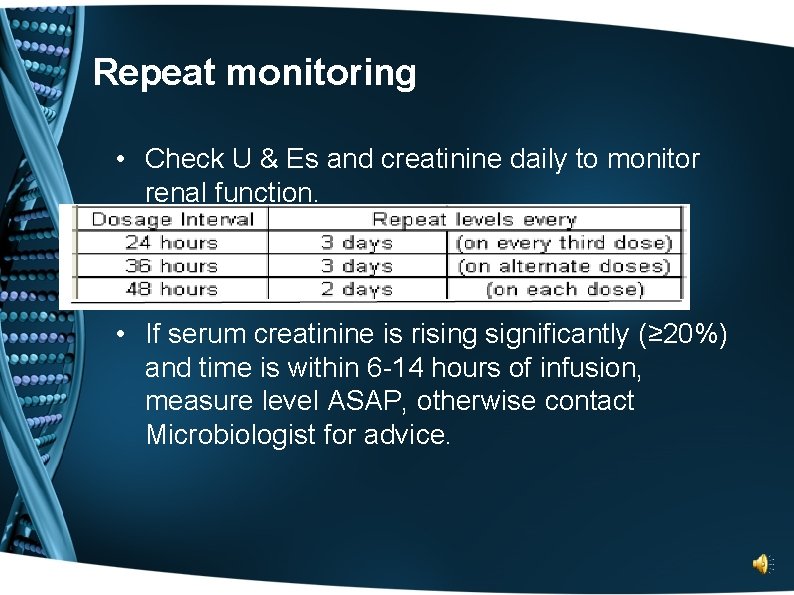
Repeat monitoring • Check U & Es and creatinine daily to monitor renal function. • If serum creatinine is rising significantly (≥ 20%) and time is within 6 -14 hours of infusion, measure level ASAP, otherwise contact Microbiologist for advice.

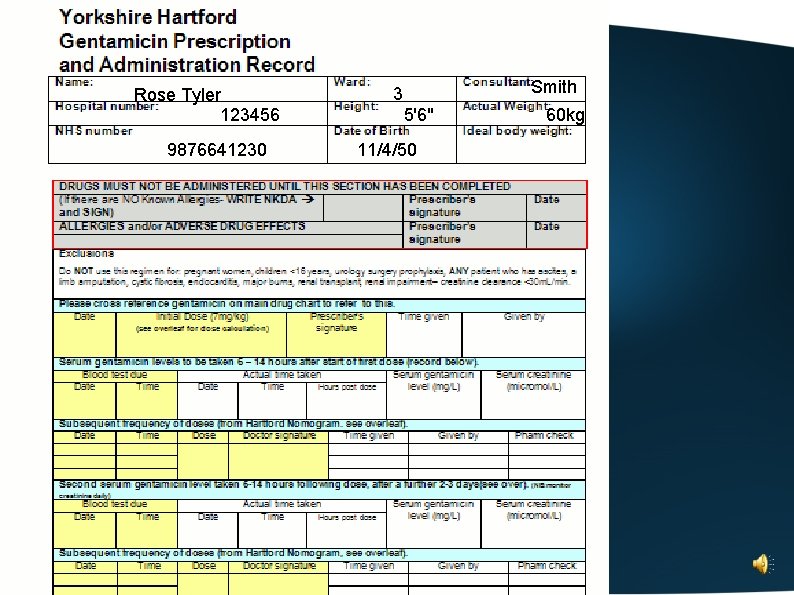
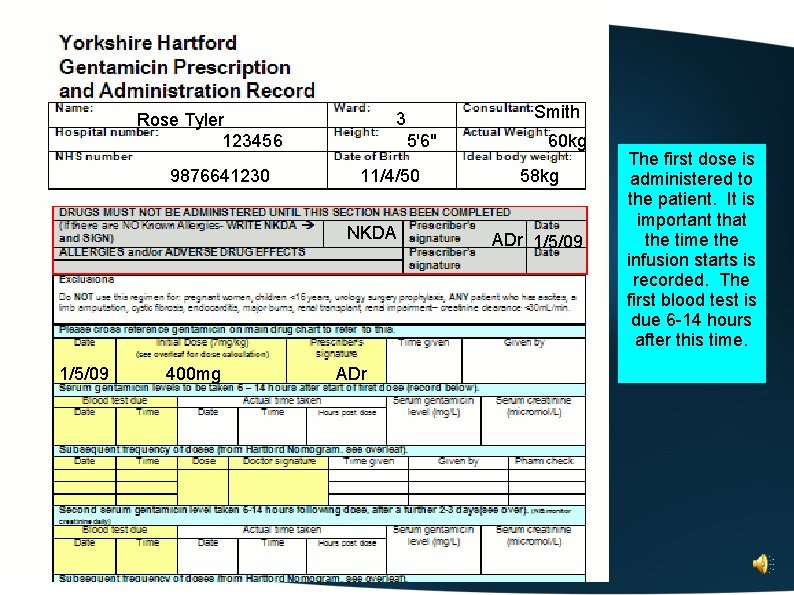
An example of how to complete the Yorkshire Hartford Gentamicin Regimen drug chart


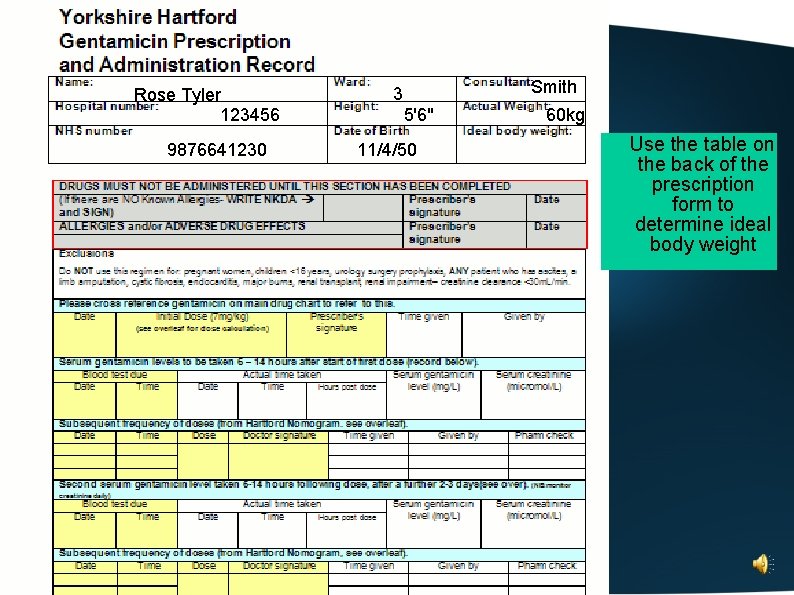
Complete the patient details at the top of the chart

Rose Tyler 123456 9876641230 Smith 3 5'6" 11/4/50 60 kg

Rose Tyler 123456 9876641230 Smith 3 5'6" 11/4/50 60 kg Use the table on the back of the prescription form to determine ideal body weight


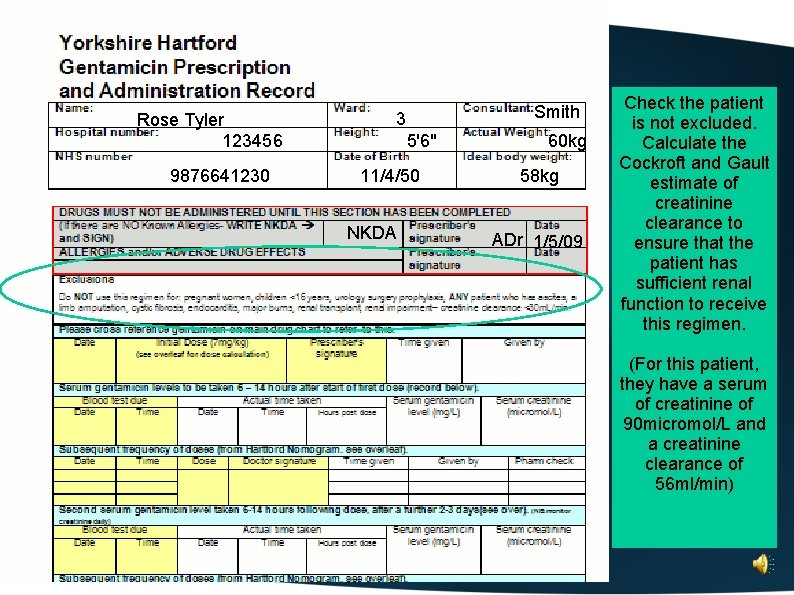
Rose Tyler 123456 9876641230 Smith 3 5'6" 11/4/50 60 kg 58 kg Complete the allergy/adverse drug effects box

Rose Tyler 123456 9876641230 Smith 3 5'6" 11/4/50 NKDA 60 kg 58 kg ADr 1/5/09 Check the patient is not excluded. Calculate the Cockroft and Gault estimate of creatinine clearance to ensure that the patient has sufficient renal function to receive this regimen. (For this patient, they have a serum of creatinine of 90 micromol/L and a creatinine clearance of 56 ml/min)

Rose Tyler 123456 9876641230 Smith 3 5'6" 11/4/50 NKDA 60 kg 58 kg ADr 1/5/09 Use the lower of the Actual Weight or Ideal Body Weight to calculate the 7 mg/kg dose, rounded to the nearest 40 mg. Alternatively, use the table on the reverse of the chart to determine the dose.

9876641230 5'6" 11/4/50 NKDA 1/5/09 400 mg Smith 3 Rose Tyler 123456 ADr 60 kg 58 kg ADr 1/5/09 The first dose is administered to the patient. It is important that the time the infusion starts is recorded. The first blood test is due 6 -14 hours after this time.

9876641230 Smith 3 Rose Tyler 123456 5'6" 11/4/50 NKDA 1/5/09 400 mg 15. 0023. 00 ADr 60 kg 58 kg ADr 1/5/09 9 am KF AS The time the sample is actually taken must be recorded.

9876641230 Smith 3 Rose Tyler 123456 5'6" 11/4/50 NKDA 1/5/09 400 mg 15. 0023. 00 1/5/09 ADr 19. 00 10 60 kg 58 kg ADr 1/5/09 9 am KF AS When the gentamicin result is available, record this along with today's serum creatinine. Serum creatinine must be measured daily.

9876641230 Smith 3 Rose Tyler 123456 11/4/50 58 kg NKDA 1/5/09 400 mg 15. 0023. 00 1/5/09 ADr 19. 00 60 kg 5'6" 10 ADr 1/5/09 9 am 5. 5 KF AS 85 Refer to microbiology or pharmacy if serum creatinine is rising sharply Plot the gentamicin level against the hours-post -dose on the chart on the back. This chart also shows when the next level is due.


9876641230 Smith 3 Rose Tyler 123456 11/4/50 58 kg NKDA 1/5/09 2/5/09 400 mg 15. 0023. 00 21: 00 09: 00 1/5/09 400 mg ADr 19. 00 ADr 60 kg 5'6" 10 ADr 1/5/09 9 am 5. 5 KF AS 85 The process continues until gentamicin is no longer required. Remember to review the need for IV antibiotics on a daily basis.

Pharmacokinetic Dosing • Also known as: – – Traditional dosing Multiple-dose-per-day dosing Tailored dosing Conventional dosing • Use for patients excluded from Hartford. • Bolus doses calculated according to estimated measures of distribution and excretion of gentamicin.

Pharmacokinetic dosing • Discuss with microbiology or pharmacy appropriate doses • Measure levels after 3 – 5 doses. • Need to monitor both peak (post-dose) and trough (pre-dose) levels. • Take pre-dose sample immediately (1 hour) before next dose, post-dose 1 hour after dose is finished.

Synergistic Dosing • Form of pharmacokinetic dosing, used in treatment of endocarditis, and other streptococcal or enterococcal infections. • Usually gentamicin plus cell wall inhibitor (e. g. benzylpenicillin, flucloxacillin or vancomycin). • Combination synergistic, so lower serum concentrations of gentamicin needed - typically peak of 3 -5 mg/L and trough of less than 1 mg/L. • Synergy only occurs when both gentamicin and cell wall inhibitor given together – hence multiple doses per day.

Synergistic Dosing • Endocarditis usually requires a minimum of 2 weeks aminoglycoside therapy, so lower serum concentrations minimise nephrotoxicity and ototoxicity. • In young patients without renal impairment, start synergistic dosing with a dose of 1 mg/kg 8 hourly. • For elderly patients / patients with poor renal function contact Pharmacist or Microbiologist for advice.

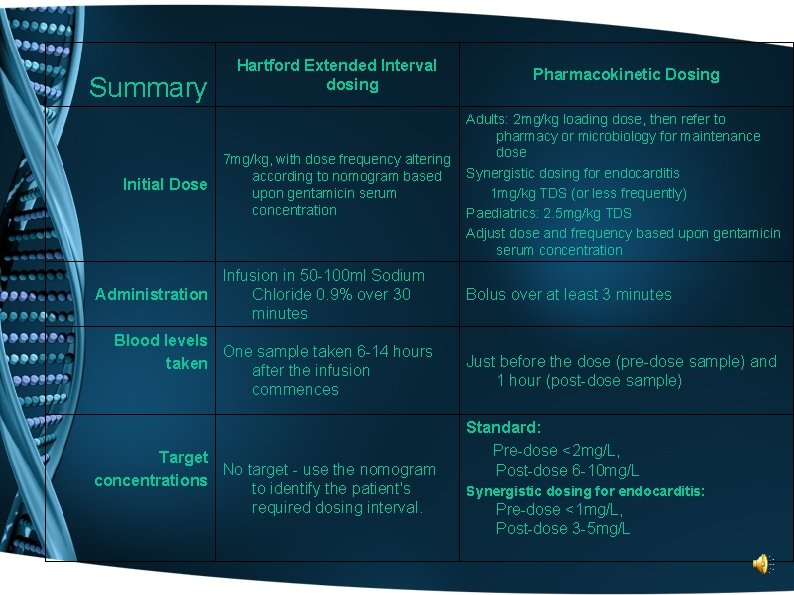
Summary Initial Dose Hartford Extended Interval dosing Pharmacokinetic Dosing 7 mg/kg, with dose frequency altering according to nomogram based upon gentamicin serum concentration Adults: 2 mg/kg loading dose, then refer to pharmacy or microbiology for maintenance dose Synergistic dosing for endocarditis 1 mg/kg TDS (or less frequently) Paediatrics: 2. 5 mg/kg TDS Adjust dose and frequency based upon gentamicin serum concentration Infusion in 50 -100 ml Sodium Chloride 0. 9% over 30 Administration minutes Blood levels One sample taken 6 -14 hours taken after the infusion commences Target No target - use the nomogram concentrations to identify the patient's required dosing interval. Bolus over at least 3 minutes Just before the dose (pre-dose sample) and 1 hour (post-dose sample) Standard: Pre-dose <2 mg/L, Post-dose 6 -10 mg/L Synergistic dosing for endocarditis: Pre-dose <1 mg/L, Post-dose 3 -5 mg/L

Credits • Yorkshire Hartford Gentamicin developed with the assistance of the Yorkshire and Humber Antimicrobial Pharmacist network – In particular: Pam Garnett, Peter Taylor, Alison Haigh, Andy Karvot and Philip Howard • Regimen developed from – Nicolau et al Experience with a Once-Daily Aminoglycoside Program Administered to 2, 184 Adult Patients. ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, 1995; 39(3): 650– 655 This Yorkshire Hartford regimen protocol and associated materials can be used, without guarantee or warranty by other healthcare professionals providing it is on a not-for-profit basis and any resulting materials are shared on a similar basis