What Does the Data Suggest for the Future

















- Slides: 36

What Does the Data Suggest for the Future Use of DCB for Coronary Application? Ron Waksman, MD Director, Cardiovascular Research Advanced Education Professor of Medicine, Georgetown University Med. Star Heart Institute

Ron Waksman, MD Consulting: Biotronik, Inc. Abbott Laboratories Boston Scientific Corporation Medtronic, Inc. Merck and Company, Inc. Honoraria: Abbott Laboratories, Boston Scientific Corporation Merck and Company, Inc. Medtronic, Inc.

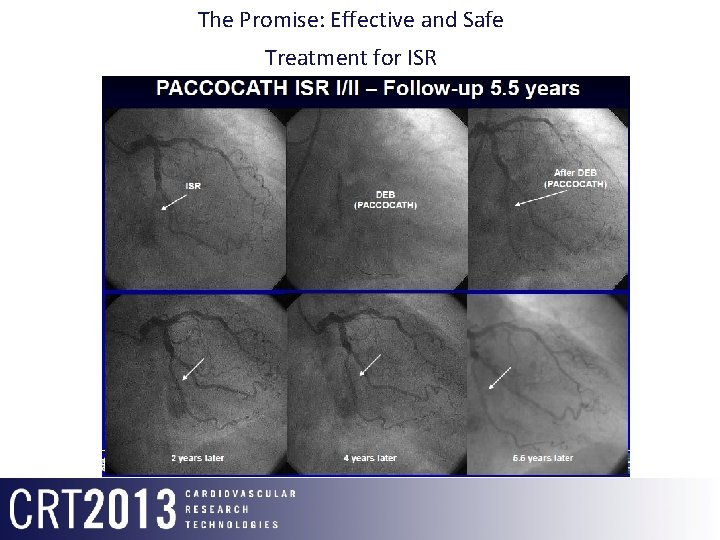
The Promise: Effective and Safe Treatment for ISR DEB (PACCOCATH) ISR 2 Years F/u 4 Years F/u

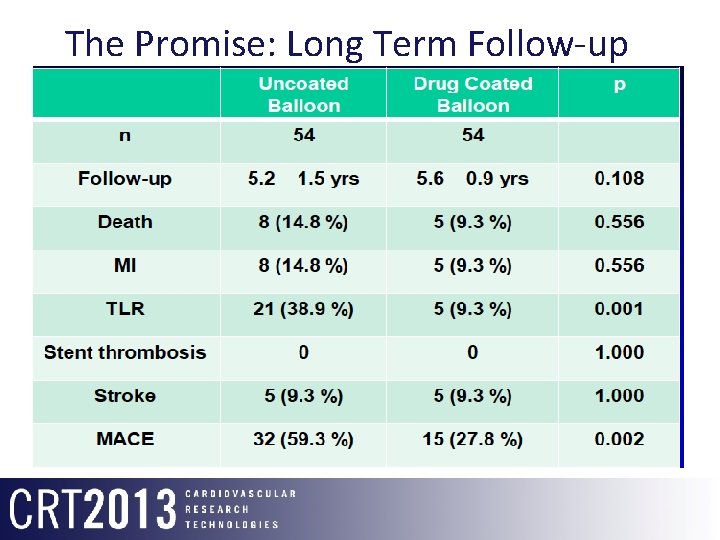
The Promise: Long Term Follow-up Pacocath ISR I/II

Pantera Lux DEB A combination of proven and novel technologies Balloon Pantera (semi-compliant) Excellent deliverability and crossability Excipient Drug Paclitaxel (3. 0 µg/mm 2) Anti-proliferative, lipophilic and allow fast absorption Butyryl-tri-hexyl citrate (BTHC) Keeps paclitaxel in micro-crystalline structure for optimal bioavailability Tested thoroughly in animal studies Showed high safety and a trend towards better efficacy compared to competitor products Pre-clinical show high safety & efficacy Pantera Lux is a potentially highly efficacious solution for treating instent restenosis, available in diameters 2. 0 – 4. 0 mm and lengths 10 – 30 mm. Product is CE marked. Not for sale in the U. S.

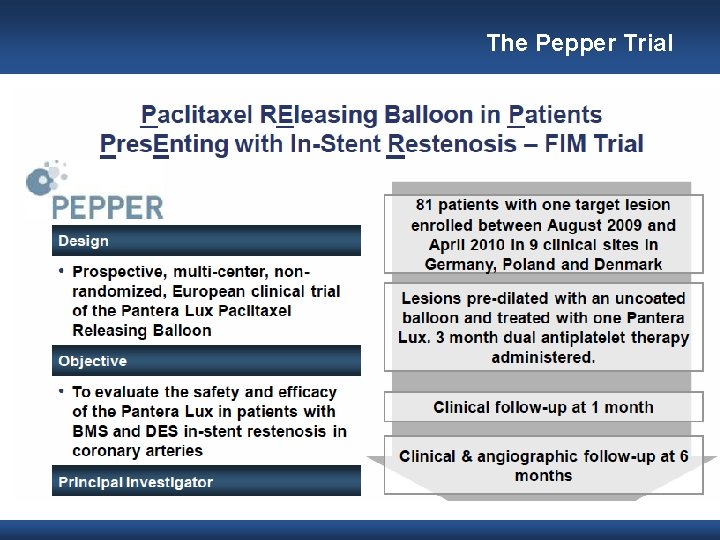
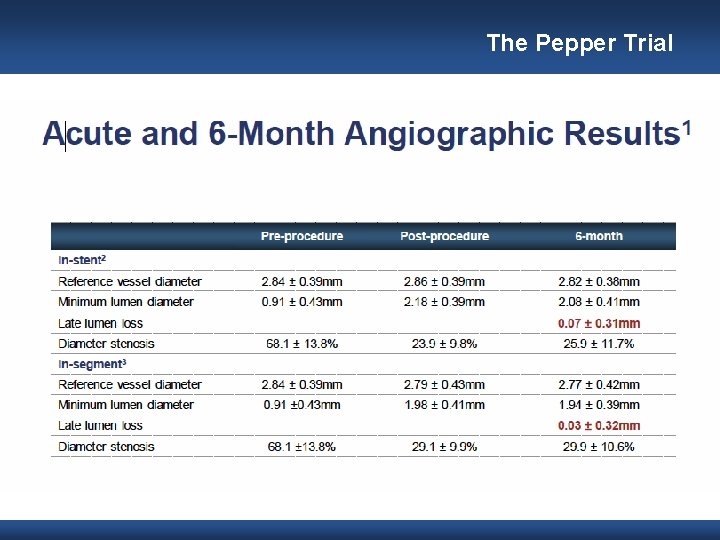
The Pepper Trial

The Pepper Trial

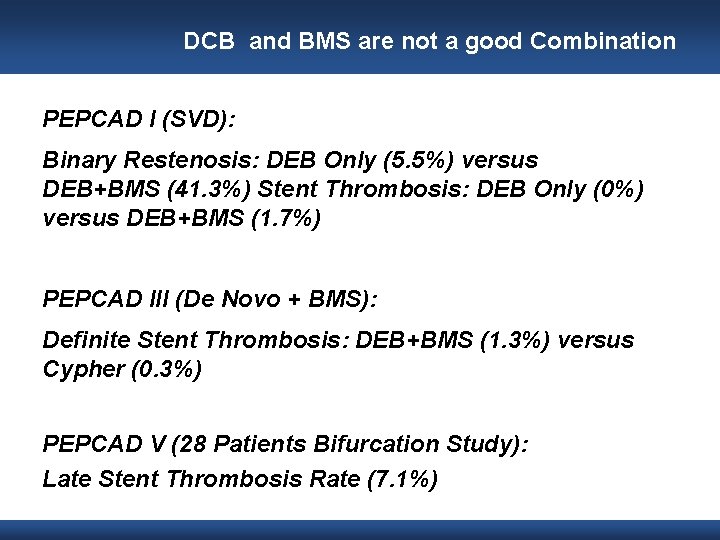
DCB and BMS are not a good Combination PEPCAD I (SVD): Binary Restenosis: DEB Only (5. 5%) versus DEB+BMS (41. 3%) Stent Thrombosis: DEB Only (0%) versus DEB+BMS (1. 7%) PEPCAD III (De Novo + BMS): Definite Stent Thrombosis: DEB+BMS (1. 3%) versus Cypher (0. 3%) PEPCAD V (28 Patients Bifurcation Study): Late Stent Thrombosis Rate (7. 1%)

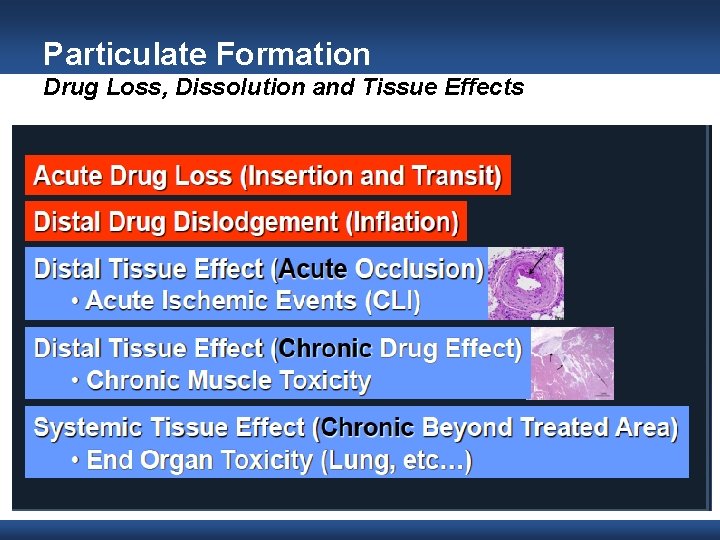
Particulate Formation Drug Loss, Dissolution and Tissue Effects

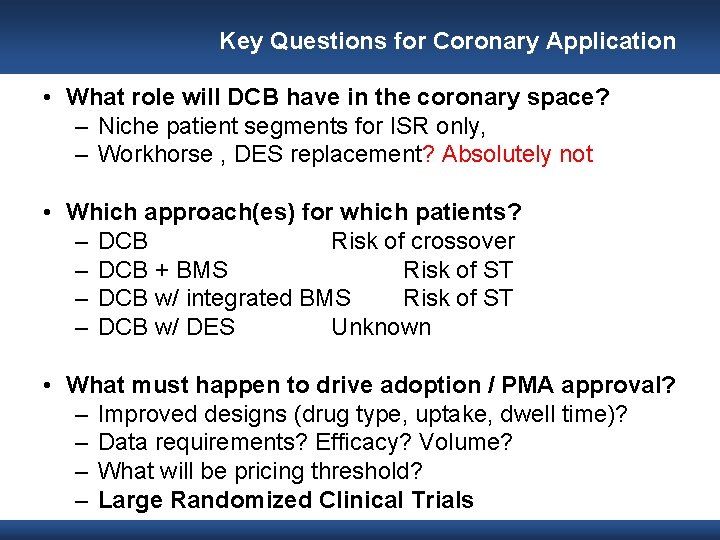
Key Questions for Coronary Application • What role will DCB have in the coronary space? – Niche patient segments for ISR only, – Workhorse , DES replacement? Absolutely not • Which approach(es) for which patients? – DCB Risk of crossover – DCB + BMS Risk of ST – DCB w/ integrated BMS Risk of ST – DCB w/ DES Unknown • What must happen to drive adoption / PMA approval? – Improved designs (drug type, uptake, dwell time)? – Data requirements? Efficacy? Volume? – What will be pricing threshold? – Large Randomized Clinical Trials

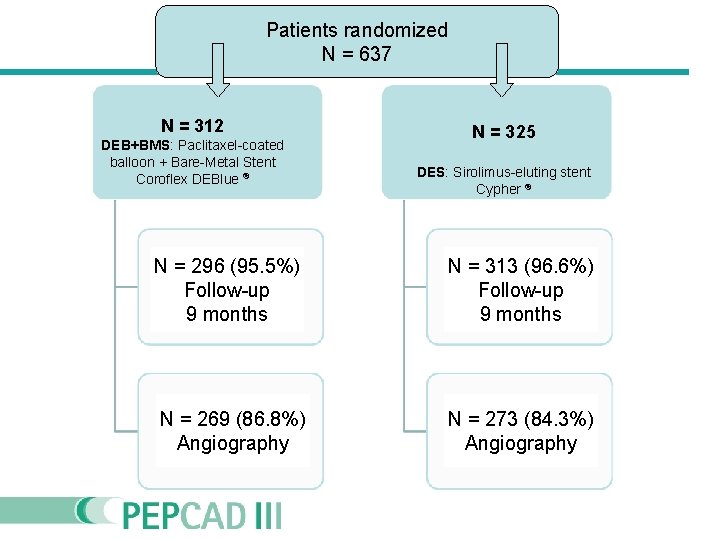
Patients randomized N = 637 N = 312 DEB+BMS: Paclitaxel-coated balloon + Bare-Metal Stent Coroflex DEBlue ® N = 325 DES: Sirolimus-eluting stent Cypher ® N = 296 (95. 5%) Follow-up 9 months N = 313 (96. 6%) Follow-up 9 months N = 269 (86. 8%) Angiography N = 273 (84. 3%) Angiography

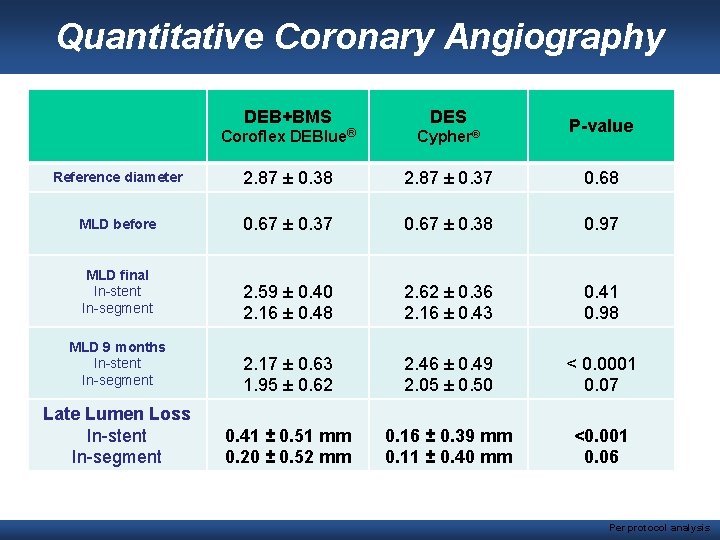
Quantitative Coronary Angiography DEB+BMS DES P-value Coroflex DEBlue® Cypher® Reference diameter 2. 87 ± 0. 38 2. 87 ± 0. 37 0. 68 MLD before 0. 67 ± 0. 37 0. 67 ± 0. 38 0. 97 2. 59 ± 0. 40 2. 16 ± 0. 48 2. 62 ± 0. 36 2. 16 ± 0. 43 0. 41 0. 98 2. 17 ± 0. 63 1. 95 ± 0. 62 2. 46 ± 0. 49 2. 05 ± 0. 50 < 0. 0001 0. 07 0. 41 ± 0. 51 mm 0. 20 ± 0. 52 mm 0. 16 ± 0. 39 mm 0. 11 ± 0. 40 mm <0. 001 0. 06 MLD final In-stent In-segment MLD 9 months In-stent In-segment Late Lumen Loss In-stent In-segment Per protocol analysis

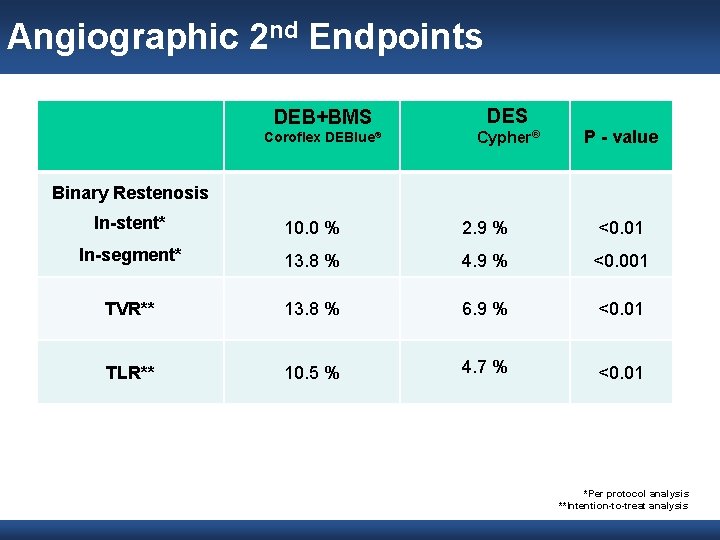
Angiographic 2 nd Endpoints DEB+BMS DES Coroflex DEBlue® Cypher® P - value Binary Restenosis In-stent* 10. 0 % 2. 9 % <0. 01 In-segment* 13. 8 % 4. 9 % <0. 001 TVR** 13. 8 % 6. 9 % <0. 01 TLR** 10. 5 % 4. 7 % <0. 01 *Per protocol analysis **Intention-to-treat analysis

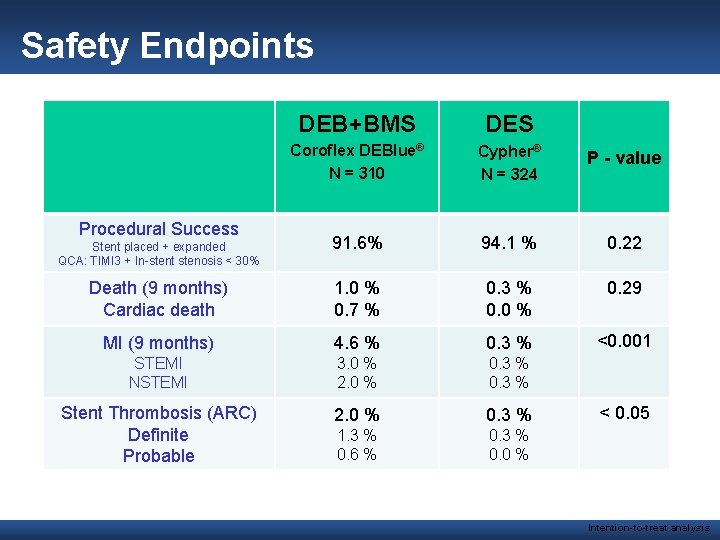
Safety Endpoints DEB+BMS DES Coroflex DEBlue® N = 310 Cypher® N = 324 P - value 91. 6% 94. 1 % 0. 22 Death (9 months) Cardiac death 1. 0 % 0. 7 % 0. 3 % 0. 0 % 0. 29 MI (9 months) 4. 6 % 0. 3 % <0. 001 STEMI NSTEMI 3. 0 % 2. 0 % 0. 3 % Stent Thrombosis (ARC) Definite Probable 2. 0 % 0. 3 % 1. 3 % 0. 6 % 0. 3 % 0. 0 % Procedural Success Stent placed + expanded QCA: TIMI 3 + In-stent stenosis < 30% < 0. 05 Intention-to-treat analysis

DIOR™-II Technology: • Paclitaxel balloon surface: 3 µg/mm² • Coating method is a 1: 1 mixture of Paclitaxel (Ph Eur. ) and Shellac (Ph Eur. ) • Protection of wash off effect: drug hidden within the balloon folds • Delivery by simple diffusion • The Coating is CE marked • Shellac is well established in Cosmetics, as food coating and Tablet coating. • Shellac is recognized as safe (GRAS) by the FDA • Balloon inflation time recommended: 20 -30 sec. @ nominal balloon pressure The optical refraction of Shellac gives balloon a shiny appearance

DEBIUT trial Late breaking clinical trial session 4 Drug Eluting Balloons in Coronary Bifurcations ; The Drug Eluting Balloon In Bif. Urcation Trial On behalf of all co-investigators By: Pieter R. Stella, MD, Ph. D University Medical Center of Utrecht The Netherlands

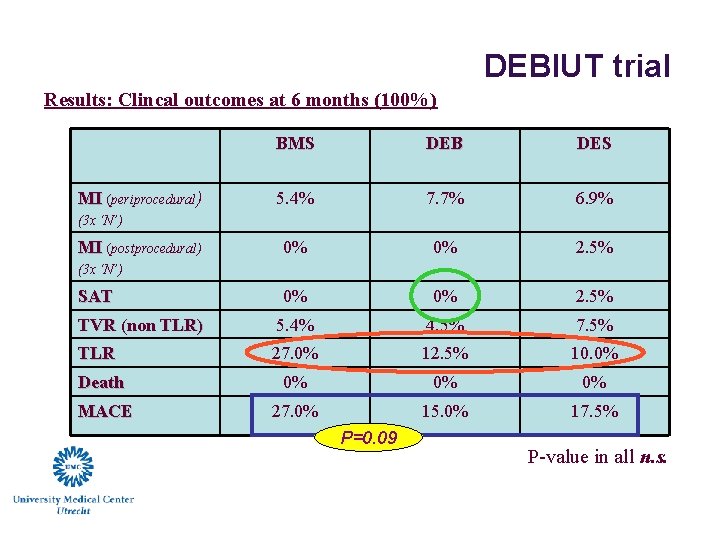
DEBIUT trial Results: Clincal outcomes at 6 months (100%) BMS DEB DES 5. 4% 7. 7% 6. 9% 0% 0% 2. 5% TVR (non TLR) 5. 4% 4. 5% 7. 5% TLR 27. 0% 12. 5% 10. 0% Death 0% 0% 0% MACE 27. 0% 15. 0% 17. 5% MI (periprocedural) (3 x ‘N’) MI (postprocedural) (3 x ‘N’) SAT P=0. 09 P-value in all n. s.

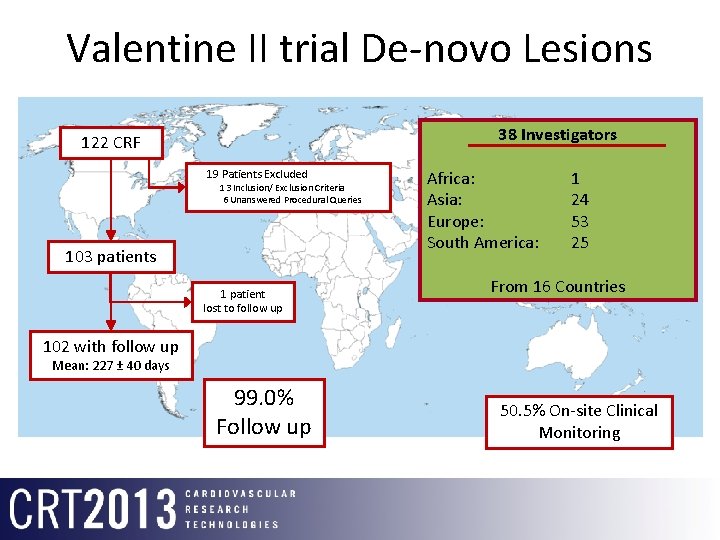
Valentine II trial De-novo Lesions 38 Investigators 122 CRF 19 Patients Excluded 13 Inclusion/ Exclusion Criteria 6 Unanswered Procedural Queries 103 patients 1 patient lost to follow up Africa: Asia: Europe: South America: 1 24 53 25 From 16 Countries 102 with follow up Mean: 227 ± 40 days 99. 0% Follow up 50. 5% On-site Clinical Monitoring

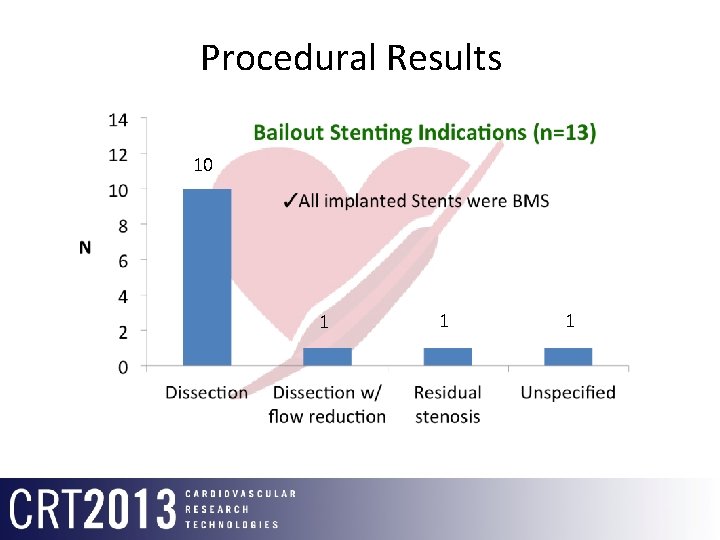
Procedural Results 10 1 1 1

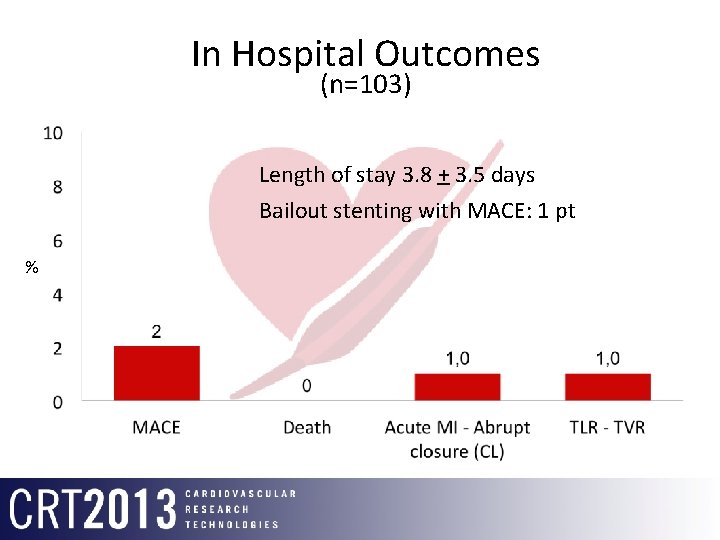
In Hospital Outcomes (n=103) Length of stay 3. 8 + 3. 5 days Bailout stenting with MACE: 1 pt %

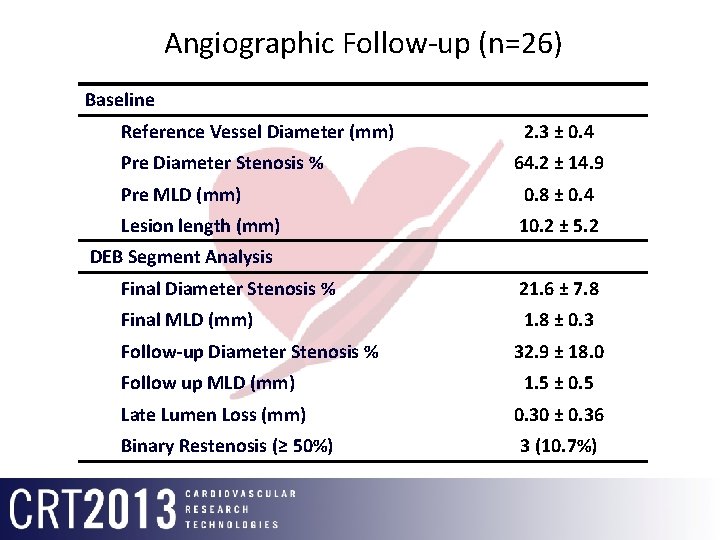
Angiographic Follow-up (n=26) Baseline Reference Vessel Diameter (mm) Pre Diameter Stenosis % 2. 3 ± 0. 4 64. 2 ± 14. 9 Pre MLD (mm) 0. 8 ± 0. 4 Lesion length (mm) 10. 2 ± 5. 2 DEB Segment Analysis Final Diameter Stenosis % 21. 6 ± 7. 8 Final MLD (mm) 1. 8 ± 0. 3 Follow-up Diameter Stenosis % 32. 9 ± 18. 0 Follow up MLD (mm) 1. 5 ± 0. 5 Late Lumen Loss (mm) 0. 30 ± 0. 36 Binary Restenosis (≥ 50%) 3 (10. 7%)

Clinical Follow-up Outcomes (n=102) % Mean Follow up was 227 ± 40 days MACE is defined as, all cause death, MI, TVR and Vessel Thrombosis










Drug Eluting Balloon in Acute Myocardial Infarction: 6 month results of the DEB-AMI study (Clinical. Trials. gov number: NCT 00856765) On behalf of the DEB-AMI study group (PI) P. R. Stella, MD, Ph. D p. stella@umcutrecht. nl

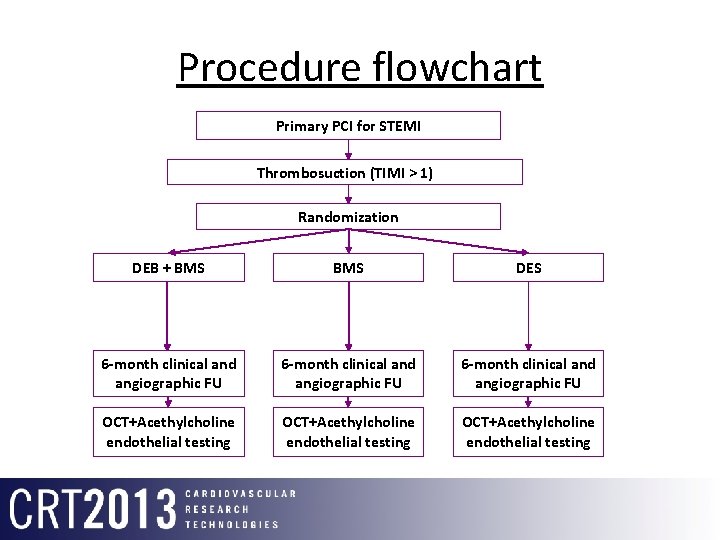
Procedure flowchart Primary PCI for STEMI Thrombosuction (TIMI > 1) Randomization DEB + BMS DES 6 -month clinical and angiographic FU OCT+Acethylcholine endothelial testing

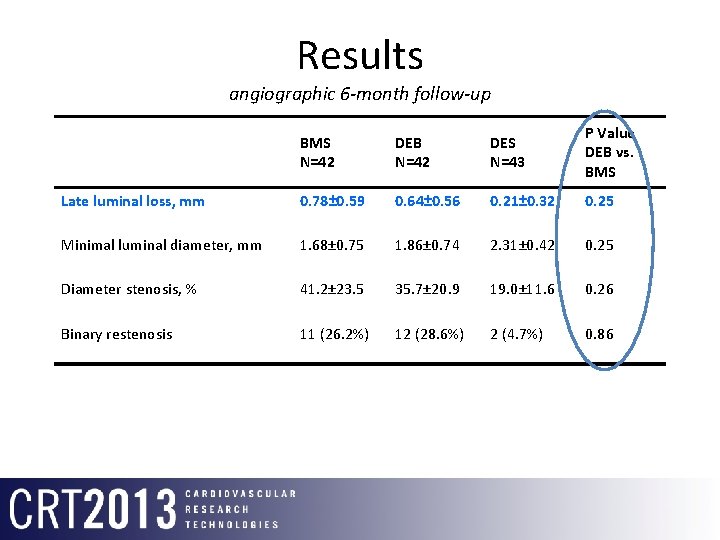
Results angiographic 6 -month follow-up BMS N=42 DEB N=42 DES N=43 P Value DEB vs. BMS Late luminal loss, mm 0. 78± 0. 59 0. 64± 0. 56 0. 21± 0. 32 0. 25 Minimal luminal diameter, mm 1. 68± 0. 75 1. 86± 0. 74 2. 31± 0. 42 0. 25 Diameter stenosis, % 41. 2± 23. 5 35. 7± 20. 9 19. 0± 11. 6 0. 26 Binary restenosis 11 (26. 2%) 12 (28. 6%) 2 (4. 7%) 0. 86

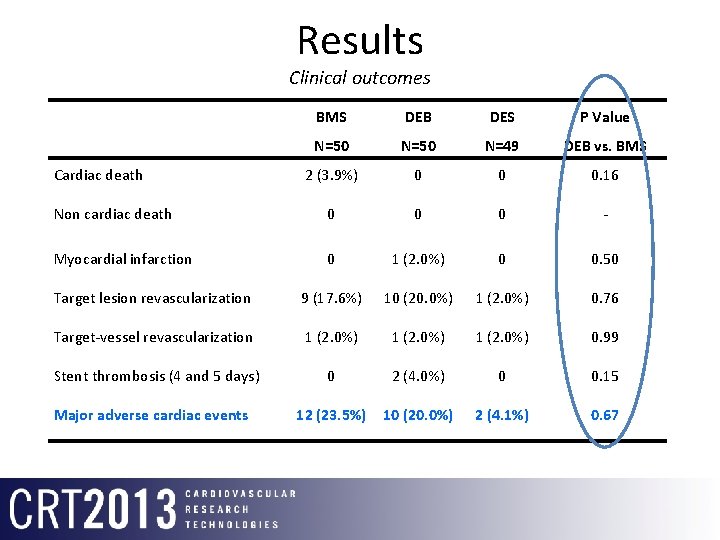
Results Clinical outcomes BMS DEB DES P Value N=50 N=49 DEB vs. BMS 2 (3. 9%) 0 0 0. 16 Non cardiac death 0 0 0 - Myocardial infarction 0 1 (2. 0%) 0 0. 50 Target lesion revascularization 9 (17. 6%) 10 (20. 0%) 1 (2. 0%) 0. 76 Target-vessel revascularization 1 (2. 0%) 0. 99 Stent thrombosis (4 and 5 days) 0 2 (4. 0%) 0 0. 15 12 (23. 5%) 10 (20. 0%) 2 (4. 1%) 0. 67 Cardiac death Major adverse cardiac events

Coated Balloons Future directions • Develop New coating to prevent Particulate release • Different drugs; Sirolimus Everolimus • Improve deliverability and profile of the balloons • Test De-novo indications when used as an adjunct therapy to POBA without stenting • Provisional bifurcation application • Redo the DEB-AMI for primary PCI without stents