Water The Universal Solvent Chapter 7 Water is


















- Slides: 24

Water: The Universal Solvent Chapter 7

Water is the only substance in nature found in abundance in solid, liquid, and gas state. Water is the main component in many foods Water is a nutrient Nutrients are _______________.

The Structure of Water Chemically composed of one oxygen atom and two hydrogen atoms = Covalent bonds Physical properties of the compound Higher melting and boiling point More surface tension Lower density Greater ability to conduct energy

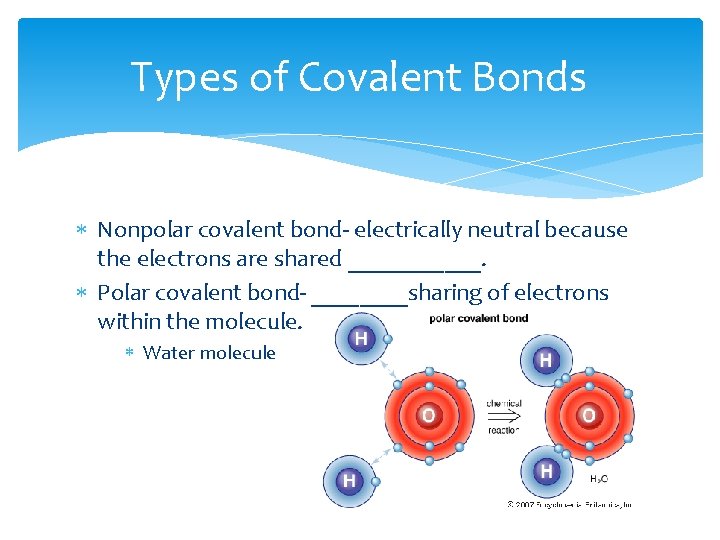
Types of Covalent Bonds Nonpolar covalent bond- electrically neutral because the electrons are shared ______. Polar covalent bond- ____sharing of electrons within the molecule. Water molecule

Hydrogen Bonds Hydrogen bond- attraction of a _____hydrogen end of one water molecule to the _____ oxygen ends of other water molecules. Hydrogen bonds are intermolecular, or _____________. Intermolecular bonds are weaker than the covalent bonds within the molecule. (ex. Holding hands) In water, the hydrogen of one atom is attracted to the oxygen of another.

Surface Tension Surface Tension- force between molecules at the outside edge of a substance. Water has greater surface tension than most compounds Glass full = convex Glass not full = concave Water is both cohesive (clings to itself) and adhesive (clings to something else as well as itself) The adhesion in water is stronger than cohesion.

Pressure, Temperature, and Phase Changes ______- Force of the weight of gases in the air pressing down on a surface. As atmospheric pressure changes, so does the boiling point of water. The higher the atmospheric pressure, the higher the boiling point. When water reaches its boiling point (100 C at sea level) it changes from a liquid to a gas.

Impact of Impurities in Water Anything added to a completely pure substance in an ______. Hot water could contain more impurities than cold water Not always unsafe Salt Can change physical and chemical characteristics Flavor ____ Boiling point Freezing point Hydrogen bonding

Functions of Water in Food Preparation 2 main functions Medium for transferring heat Cooking pasta Necessary ingredient forming many food mixtures Baked goods (acts as leavening agent)

Cooking With Steam 2 Advantages: _______ More nutritious To produce steam, water molecules have to absorb more energy. Will take slightly longer to cook with steam rather than boiling water, unless under pressure. Boiling water and steam = same temperature.

The Most Common Solvent WATER! The following are all mixtures of substances dissolved in water Beverages Candies Baked goods Soups Stews Casseroles Sauces

Gas-in-Water Solutions Ex. - Carbonated beverages Carbon dioxide Ex. - Cold water _____ Hot water has less oxygen because when water is boiled the oxygen escapes into the atmosphere. This would leave the water with a flat taste.

Liquid-in-Water Solutions Alcoholic beverages Vinegar in water (pickle juice) Fruit concentrates

Solid-in-Water Solutions Sugar and salt solutions Two most common solids Cause water to freeze at lower temp. and boil at higher Sugar is used in much higher concentrations than salt Tea and Coffee Dissolves flavor compounds Increasing the brewing time will increase the strength of flavor Ideal brewing temp is just below boiling point _______ create bitter aftertaste.

Water Content in Foods Water is a major part of most foods. Water Activity (Aw): the measure of partial water pressure over food. Supports enzyme activity. Water becomes part of the structure of food in 3 main ways Free water: can be easily separated from food tissues. Ex: grapefruit juice, lemon juice Bound water: tied to the structure of large molecules. Fruits, meats, vegetables Hydrate: any chemical compound that is loosely bound with water. Caffeine molecule

Water Content in Food Broccoli (cooked), 91% water Cheese Pizza (baked), 45% water Eggs (raw), 75% water Brownies, 10% water

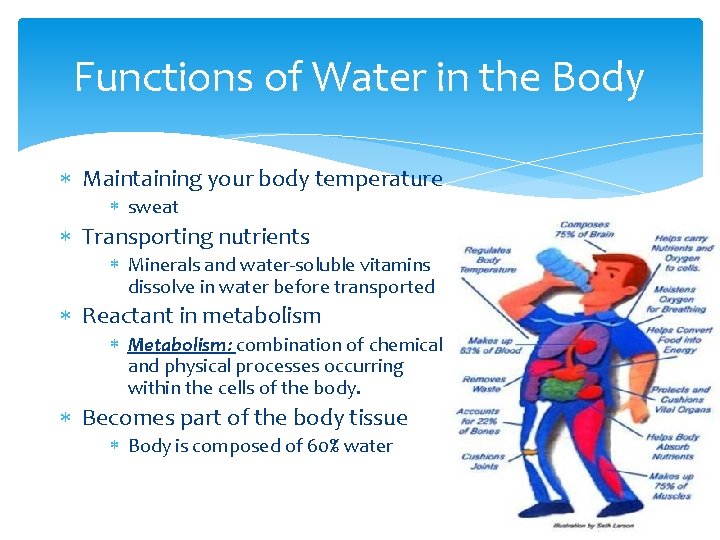
Functions of Water in the Body Maintaining your body temperature sweat Transporting nutrients Minerals and water-soluble vitamins dissolve in water before transported Reactant in metabolism Metabolism: combination of chemical and physical processes occurring within the cells of the body. Becomes part of the body tissue Body is composed of 60% water

Meeting Your Body’s Water Needs 6 -8 glasses of water per day. A person can live much longer without food than without water, but why can people who never drink water survive? All foods are made up of water. Many foods that are high in water are also high in sugar and salt More healthful to drink plain water than other beverages as a water source because water has no calories. Even diet soft drinks are bad because they contain excess sugars and phosphorous which can keep the body from absorbing other nutrients.

The Role of Thirst It is best to consume water regularly throughout the day. After drinking sweetened beverages, thirst will occur in about 30 minutes. Ice water relieves thirst faster than warm water. Constricts the stomach, forces water into the bloodstream faster. • Fun fact: More water evaporates from skin and breath in high altitudes, where atmospheric pressure is low. So, if you are flying in an airplane you will need more water than normal to keep you hydrated.

A Safe Water Supply Water is the most valuable resource because all life depends on it. Since water is recycled through collected rainwater, lakes and rivers, and underground water, We had to find a way to keep it safe and clean. Used to have many problems: Typhoid caused by bacteria from feces in water The Mediterranean Sea is toxic to most life-forms due to factory and human waste In the 70’s lake Erie was thought to be “dead” because the toxic buildup was so bad.

Biological Pollutants Pollutant: Anything that makes a substance impure. Pollutants include bacteria, protozoa, viruses, and organic waste Large volumes of microbes can deplete the oxygen levels in lakes and rivers. Cause fish to suffocate Typhoid and Cholera are water-borne diseases caused by bacteria spread from human and animal feces.

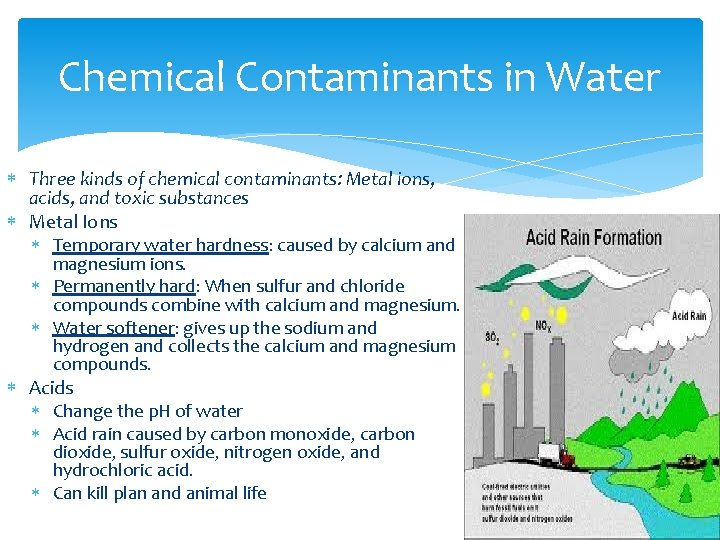
Chemical Contaminants in Water Three kinds of chemical contaminants: Metal ions, acids, and toxic substances Metal Ions Temporary water hardness: caused by calcium and magnesium ions. Permanently hard: When sulfur and chloride compounds combine with calcium and magnesium. Water softener: gives up the sodium and hydrogen and collects the calcium and magnesium compounds. Acids Change the p. H of water Acid rain caused by carbon monoxide, carbon dioxide, sulfur oxide, nitrogen oxide, and hydrochloric acid. Can kill plan and animal life

Physical Contaminants in Water Garbage Litter Animals and fish die from both toxins and swallowing plastics.

Water Contaminants in the Beverage Industry Water has different contaminants in different parts of the world. Companies have established water standards. Remove contaminants from water before adding soft drink syrups.