Water the Universal Solvent Integrated Physics and Chemistry




- Slides: 9


Water the Universal Solvent Integrated Physics and Chemistry (9) Science Concepts. The student knows how solution chemistry is a part of everyday life. The student is expected to (A) relate the structure of water to its function [as the universal solvent];

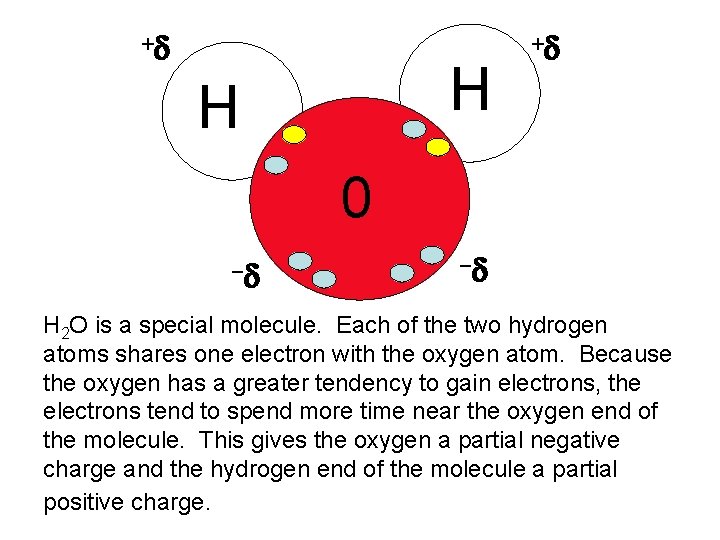
+d H H +d 0 -d -d H 2 O is a special molecule. Each of the two hydrogen atoms shares one electron with the oxygen atom. Because the oxygen has a greater tendency to gain electrons, the electrons tend to spend more time near the oxygen end of the molecule. This gives the oxygen a partial negative charge and the hydrogen end of the molecule a partial positive charge.

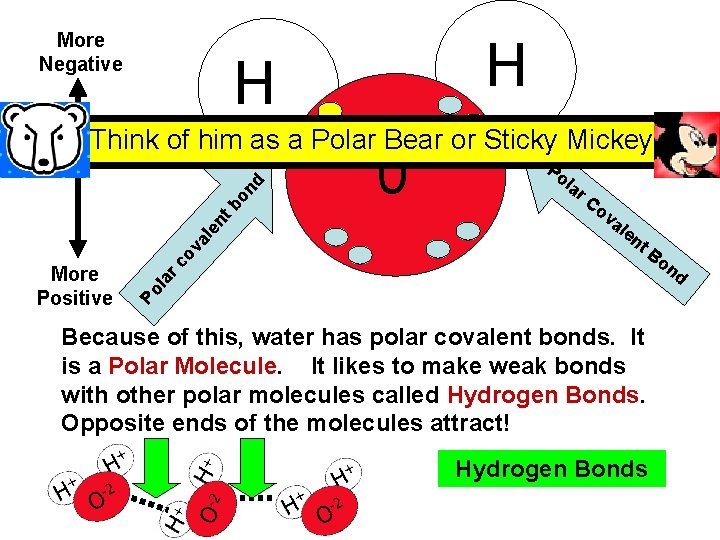
More Negative H H 0 Po la r. C ov al en t. B on d Po la More Positive rc ov al en tb on d Think of him as a Polar Bear or Sticky Mickey Because of this, water has polar covalent bonds. It is a Polar Molecule. It likes to make weak bonds with other polar molecules called Hydrogen Bonds. Opposite ends of the molecules attract! Hydrogen Bonds

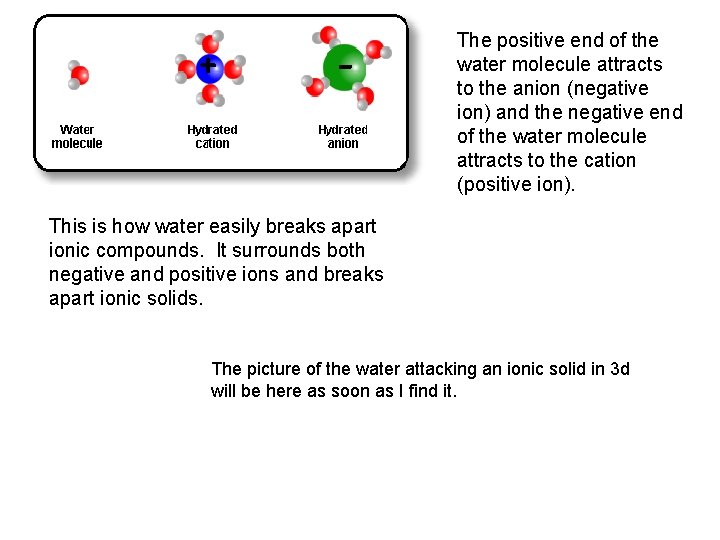
+ - The positive end of the water molecule attracts to the anion (negative ion) and the negative end of the water molecule attracts to the cation (positive ion). This is how water easily breaks apart ionic compounds. It surrounds both negative and positive ions and breaks apart ionic solids. The picture of the water attacking an ionic solid in 3 d will be here as soon as I find it.

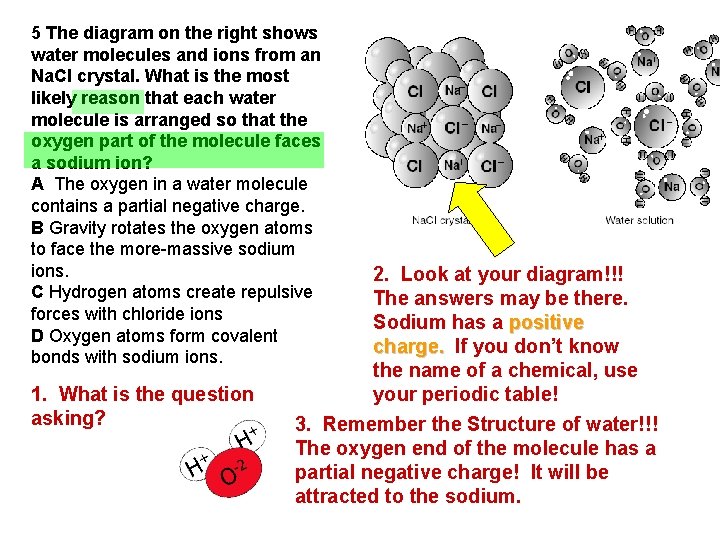
5 The diagram on the right shows water molecules and ions from an Na. Cl crystal. What is the most likely reason that each water molecule is arranged so that the oxygen part of the molecule faces a sodium ion? A The oxygen in a water molecule contains a partial negative charge. B Gravity rotates the oxygen atoms to face the more-massive sodium ions. C Hydrogen atoms create repulsive forces with chloride ions D Oxygen atoms form covalent bonds with sodium ions. 1. What is the question asking? 2. Look at your diagram!!! The answers may be there. Sodium has a positive charge. If you don’t know the name of a chemical, use your periodic table! 3. Remember the Structure of water!!! The oxygen end of the molecule has a partial negative charge! It will be attracted to the sodium.

You have been given a water molecule. Let’s play… 1. Simon Says: Hydrogen bond with two of your neighbors. 2. Simon Says: Point the correct end of your water molecule to this ion: Na+1 3. Simon Says: Point the correct end of your water molecule to this ion: Cl-1 4. Simon Says: Point the correct end of your water molecule to this ion: SO 4 -2 5. Simons Says: Point the correct end of your water molecule to this ion: Ca 2+ 6. Point the correct end of your water molecule to this molecule:


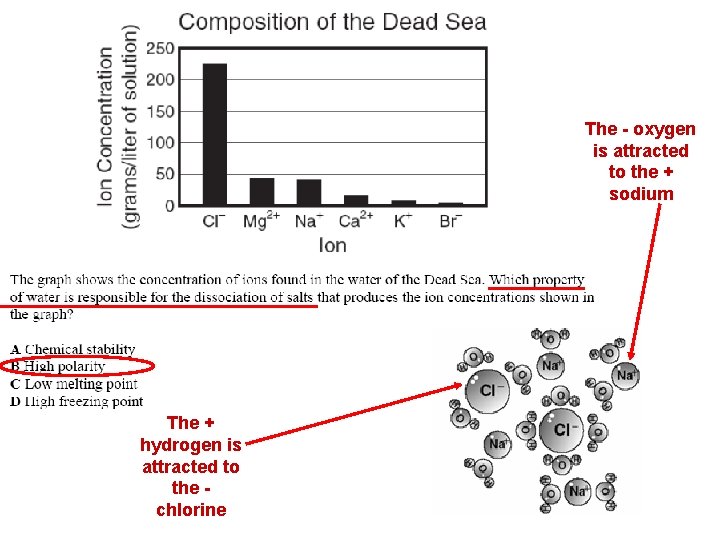
The - oxygen is attracted to the + sodium The + hydrogen is attracted to the chlorine