Walsall Medicines of Limited Clinical Value Interactive Guidelines



















![References • Coeliac UK https: //www. coeliac. org. uk/home/ Accessed]14. 11. 2016 • Electronic References • Coeliac UK https: //www. coeliac. org. uk/home/ Accessed]14. 11. 2016 • Electronic](https://slidetodoc.com/presentation_image_h2/56f0ae1e0d24d5c83fdc616c55e761e4/image-31.jpg)
- Slides: 31

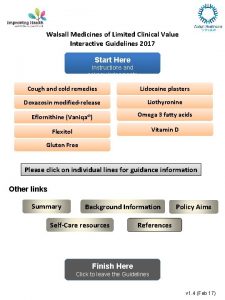
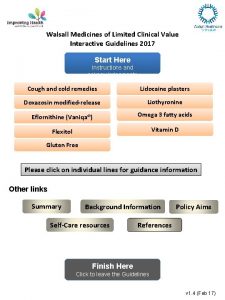
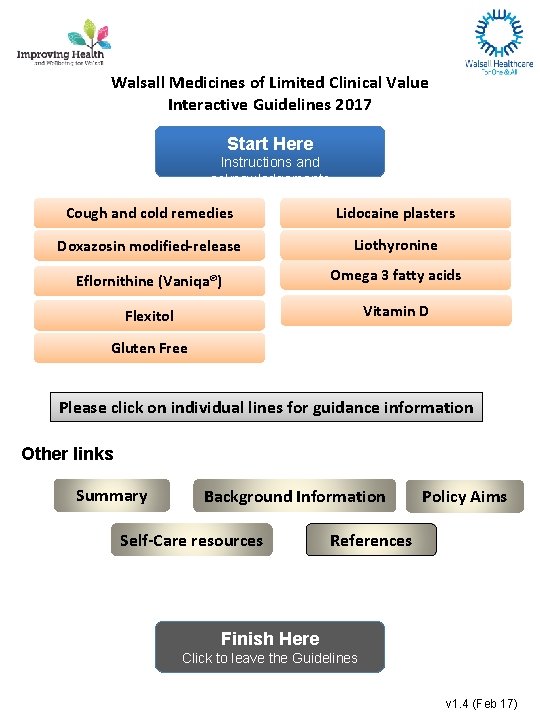
Walsall Medicines of Limited Clinical Value Interactive Guidelines 2017 Start Here Instructions and acknowledgements Cough and cold remedies Lidocaine plasters Doxazosin modified-release Liothyronine Eflornithine (Vaniqa®) Omega 3 fatty acids Flexitol Vitamin D Gluten Free Please click on individual lines for guidance information Other links Summary Background Information Self-Care resources Policy Aims References Finish Here Click to leave the Guidelines v 1. 4 (Feb 17)

Walsall CCG Policy Guidance MEDICINES OF LIMITED CLINICAL VALUE Introduction These guidelines are intended to assist healthcare professionals when prescribing Medicines of Limited Clinical Value. They have been ratified by the Walsall Joint Medicines Management Committee (JMMC in February 2017. These guidelines are based on the best available evidence but their application can always be modified by professional judgement. Cough and cold remedies Doxazosin modified-release Eflornithine (Vaniqa®) Flexitol Acknowledgments Many thanks to Dudley CCG, Sandwell and West Birmingham CCG and Wolverhampton CCG, in addition to all health care professionals and patient representatives who have inputted to and commented on this guideline. Gluten Free Lidocaine plasters Liothyronine Omega 3 fatty acids Vitamin D Run as a slide show only. Clicking on the coloured boxes in the slides e. g. will direct you to the part of the guidelines that you have selected. Click on the “Return to start” box to return to the Front Page Use your mouse in the presentation Do not use the slide controls or return and back buttons as these will operate the normal Power. Point actions. Please click on individual lines for guidance information Self-Care resources Return to start

Walsall CCG Policy Guidance MEDICINES OF LIMITED CLINICAL VALUE Summary Walsall CCG advises that only treatments which are clinically effective and provide a clear health benefit to patients should be prescribed on NHS prescriptions. Clinicians have a responsibility to only prescribe medicines that are known to be clinically effective and provide a health benefit to the patient. Background Aims References Return to start

Walsall CCG Policy Guidance MEDICINES OF LIMITED CLINICAL VALUE Background Medicines optimisation is key to achieving the best outcomes for patients. The Royal Pharmaceutical Society good practice guide on medicines optimisation outlines principles to help patients get the most out of their medicines. Walsall CCG has reviewed the prescribing of medicines and prescribable products available on the NHS in line with these principles and has decided that medicines deemed to be of limited or no clinical value and should not be routinely prescribed for adults and children within Walsall are referred to as “Medicines of Limited Clinical Value” (MOLCV). Walsall CCG is keen to ensure that only treatments that are clinically effective and provide a clear health benefit to patients are prescribed on NHS prescriptions. This is to ensure that CCG resources provide interventions with a proven health gain for the population. Therefore Walsall CCG recommends that clinicians prioritise resources based on evidence of the clinical effectiveness and safety of treatments, their cost effectiveness, and on which interventions provide the best health outcomes. In the case of treatments which can be prescribed on NHS prescriptions, the CCG is reviewing treatments that provide limited health benefit. They should be considered a LOW PRIORITY and not suitable for prescribing unless patients fall into an exception category. Some medicines that are used to treat minor ailments do not require the patient to be seen by a GP or Nurse employed within General Practice. Self-care products can be purchased from pharmacies and supermarkets. Pharmacists and other trained staff members are experts on providing advice around minor ailments; they are easy to access without an appointment and most local pharmacies provide the Minor Ailments Scheme (MAS), known locally as the Pharmacy First Scheme. Use of the MAS will free up GP time to see more complex patients. Click HERE for list of providers. Some other products are clinically ineffective or not cost effective. These treatments will not have undergone rigorous clinical trials to demonstrate that they are effective. It is inappropriate to direct NHS resources towards products that do not have proven efficacy or safety, i. e. unlicensed medicines. Walsall CCG encourages clinicians to refer to the local health economy formulary and guidelines: http: //walsallccg. nhs. uk/publications Clinicians will be required to consider whether the benefit of prescribing a treatment for an individual justifies the expense to the NHS. Such judgements should be based purely on clinical factors and should not be influenced by socio-economic aspects such as the patient’s ability to purchase. It is best practice to clearly document any decisions made on the clinical systems. Return to start

Walsall CCG Policy Guidance MEDICINES OF LIMITED CLINICAL VALUE Aims The aim of this guidance is to ensure that: • The prescribing of Medicines of Limited Clinical Value is not made routine • To support the review of the prescribing of Medicines of Limited Clinical Value • The CCG prescribing budget is utilised on evidence based medicines and interventions. • Patients will be supported to understand more about their medicines and to make choices about prevention, self-care and healthy living, where possible being directed to Community Pharmacy who can provide advice on medicines and supply either over the counter or via the Minor Ailments Scheme (Pharmacy First Scheme). • It becomes routine practice to signpost patients to further help with their medicines and to local patient support groups. Return to start

Signposting resources Patients may be directed to the following resources to help support self-care. NHS CHOICES: http: //www. nhs. uk/pages/home. aspx PATIENT. CO. UK http: //patient. info/ Self-care forum http: //www. selfcareforum. org/ Self-care section on Walsall CCG website http: //walsallccg. nhs. uk/stay-well-walsall Return to start

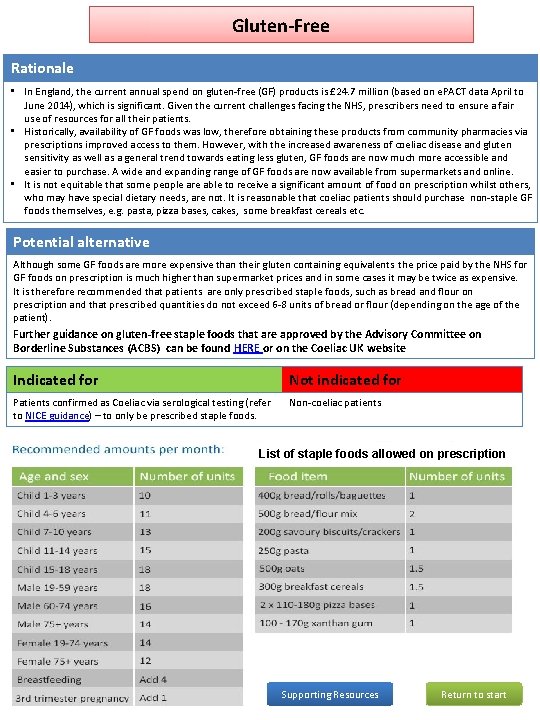
Gluten-Free Rationale • In England, the current annual spend on gluten-free (GF) products is £ 24. 7 million (based on e. PACT data April to June 2014), which is significant. Given the current challenges facing the NHS, prescribers need to ensure a fair use of resources for all their patients. • Historically, availability of GF foods was low, therefore obtaining these products from community pharmacies via prescriptions improved access to them. However, with the increased awareness of coeliac disease and gluten sensitivity as well as a general trend towards eating less gluten, GF foods are now much more accessible and easier to purchase. A wide and expanding range of GF foods are now available from supermarkets and online. • It is not equitable that some people are able to receive a significant amount of food on prescription whilst others, who may have special dietary needs, are not. It is reasonable that coeliac patients should purchase non-staple GF foods themselves, e. g. pasta, pizza bases, cakes, some breakfast cereals etc. Potential alternative Although some GF foods are more expensive than their gluten containing equivalents the price paid by the NHS for GF foods on prescription is much higher than supermarket prices and in some cases it may be twice as expensive. It is therefore recommended that patients are only prescribed staple foods, such as bread and flour on prescription and that prescribed quantities do not exceed 6 -8 units of bread or flour (depending on the age of the patient). Further guidance on gluten-free staple foods that are approved by the Advisory Committee on Borderline Substances (ACBS) can be found HERE or on the Coeliac UK website Indicated for Not indicated for Patients confirmed as Coeliac via serological testing (refer to NICE guidance) – to only be prescribed staple foods. Non-coeliac patients List of staple foods allowed on prescription Supporting Resources Return to start

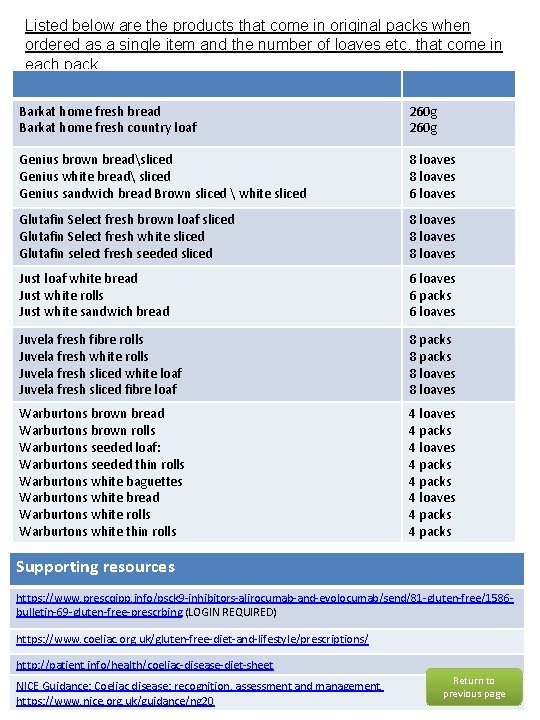
Listed below are the products that come in original packs when ordered as a single item and the number of loaves etc. that come in each pack. Barkat home fresh bread Barkat home fresh country loaf 260 g Genius brown breadsliced Genius white bread sliced Genius sandwich bread Brown sliced white sliced 8 loaves 6 loaves Glutafin Select fresh brown loaf sliced Glutafin Select fresh white sliced Glutafin select fresh seeded sliced 8 loaves Just loaf white bread Just white rolls Just white sandwich bread 6 loaves 6 packs 6 loaves Juvela fresh fibre rolls Juvela fresh white rolls Juvela fresh sliced white loaf Juvela fresh sliced fibre loaf 8 packs 8 loaves Warburtons brown bread Warburtons brown rolls Warburtons seeded loaf: Warburtons seeded thin rolls Warburtons white baguettes Warburtons white bread Warburtons white rolls Warburtons white thin rolls 4 loaves 4 packs 4 loaves 4 packs Supporting resources https: //www. prescqipp. info/psck 9 -inhibitors-alirocumab-and-evolocumab/send/81 -gluten-free/1586 bulletin-69 -gluten-free-prescrbing (LOGIN REQUIRED) https: //www. coeliac. org. uk/gluten-free-diet-and-lifestyle/prescriptions/ http: //patient. info/health/coeliac-disease-diet-sheet NICE Guidance: Coeliac disease: recognition, assessment and management. https: //www. nice. org. uk/guidance/ng 20 Return to previous page

Lidocaine Patches Rationale • Prescribing of the lidocaine 5% plaster in primary care should be RESTRICTED to patients diagnosed with postherpetic neuralgia (PHN), in whom alternative treatments have proved ineffective or where such treatments are contraindicated. • Lidocaine patches are used to relieve the pain of post-herpetic neuralgia (PHN; the burning, stabbing pains, or aches that may last for months or years after a shingles infection). • Lidocaine is in a class of medications called local anaesthetics. It works by stopping nerves from sending pain signals Potential alternative See Nice evidence for “Management of neuropathic pain “March 2010. Pregabalin or Amitriptyline for non-diabetic neuropathic pain Opioid based analgesia such as Tramadol can be used as third line or in combination with others. Amitriptyline Gabapentin Pregabalin Duloxetine Indicated for Not indicated for Symptomatic relief of neuropathic pain associated with previous herpes zoster infection. Any other pain management Walsall Healthcare Guide to Prescribing Analgesics for Non-Malignant Chronic Pain Supporting resources Patient Letter Pres. QIPP Bulletin (Nov 13): Lidocaine Plasters MTRAC Verdict and Summary Review: (May 10) Lidocaine 5% plaster NICE CG 173 (March 2010) Neuropathic pain in adults: pharmacological management in non-clinical settings Return to start

IMPORTANT INFORMATION ABOUT YOUR MEDICATION Dear (Insert patients name) We are currently reviewing the usage of lidocaine Plasters, also known as Versatis® which has been prescribed for the treatment of pain. We have undertaken an audit of our prescribing of lidocaine plasters and have identified that you have not had your prescription reviewed recently. Please contact the surgery on the number below to arrange an appointment for a review of your medicines. Surgery telephone number 01922 xxxxxx Yours sincerely Please not that all patient letters are available as a separate Word document. If you do not have access to these, please contact your practice pharmacist. Return to Lidocaine plasters guidance Return to start

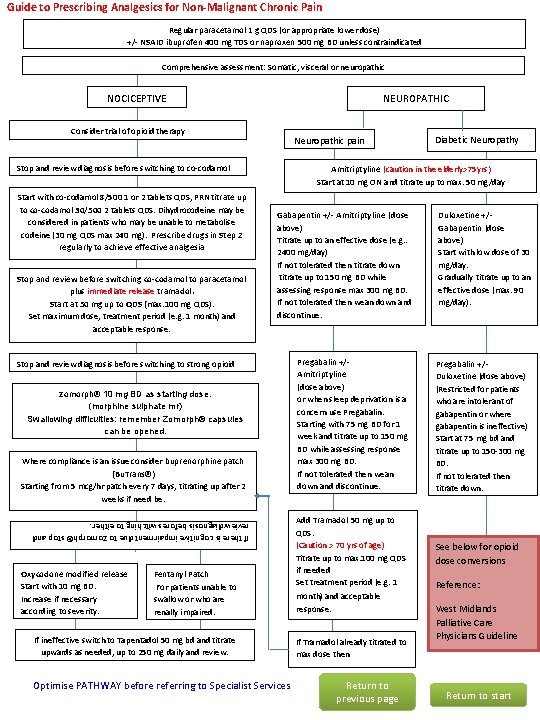
Guide to Prescribing Analgesics for Non-Malignant Chronic Pain Regular paracetamol 1 g QDS (or appropriate lower dose) +/- NSAID ibuprofen 400 mg TDS or naproxen 500 mg BD unless contraindicated Comprehensive assessment: Somatic, visceral or neuropathic NEUROPATHIC NOCICEPTIVE Consider trial of opioid therapy Neuropathic pain Stop and review diagnosis before switching to co-codamol Start with co-codamol 8/500 1 or 2 tablets QDS, PRN titrate up to co-codamol 30/500 2 tablets QDS. Dihydrocodeine may be considered in patients who may be unable to metabolise codeine (30 mg QDS max 240 mg). Prescribe drugs in Step 2 regularly to achieve effective analgesia Stop and review before switching co-codamol to paracetamol plus immediate release tramadol. Start at 50 mg up to QDS (max. 100 mg QDS). Set maximum dose, treatment period (e. g. 1 month) and acceptable response. Amitriptyline (caution in the elderly>75 yrs) Start at 10 mg ON and titrate up to max. 50 mg/day Gabapentin +/- Amitriptyline (dose above) Titrate up to an effective dose (e. g. . 2400 mg/day) If not tolerated then titrate down titrate up to 150 mg BD while assessing response max 300 mg BD. If not tolerated then wean down and discontinue. Stop and review diagnosis before switching to strong opioid Zomorph® 10 mg BD as starting dose. (morphine sulphate mr) Swallowing difficulties: remember Zomorph® capsules can be opened. Where compliance is an issue consider buprenorphine patch (Bu. Trans®) Starting from 5 mcg/hr patch every 7 days, titrating up after 2 weeks if need be. Pregabalin +/Amitriptyline (dose above) or when sleep deprivation is a concern use Pregabalin. Starting with 75 mg BD for 1 week and titrate up to 150 mg BD while assessing response max 300 mg BD. If not tolerated then wean down and discontinue. Fentanyl Patch For patients unable to swallow or who are renally impaired. Add Tramadol 50 mg up to QDS. (Caution > 70 yrs of age) Titrate up to max. 100 mg QDS if needed Set treatment period (e. g. 1 month) and acceptable response. If ineffective switch to Tapentadol 50 mg bd and titrate upwards as needed, up to 250 mg daily and review. If Tramadol already titrated to max dose then If there is cognitive impairment due to Zomorph® stop and review diagnosis before switching to either. Oxycodone modified release Start with 10 mg BD. Increase if necessary according to severity. Diabetic Neuropathy Optimise PATHWAY before referring to Specialist Services Return to previous page Duloxetine +/Gabapentin (dose above) Start with low dose of 30 mg/day. Gradually titrate up to an effective dose (max. 90 mg/day). Pregabalin +/Duloxetine (dose above) (Restricted for patients who are intolerant of gabapentin or where gabapentin is ineffective) Start at 75 mg bd and titrate up to 150 -300 mg BD. If not tolerated then titrate down. See below for opioid dose conversions Reference: West Midlands Palliative Care Physicians Guideline Return to start

Flexitol is NOT on Walsall CCG Formulary List. RATIONALE There is limited evidence for the use of hydrating creams in the treatment and management of diabetics with severe xerosis (severe dry skin). Any patient with non-severe dry skin who requests Flexitol Heel™ balm or other hydrating creams (i. e. Dermatonics, Calmurid™ etc. ) should be advised to purchase themselves. It should not be prescribed on the NHS for cosmetic purposes. You may wish to treat diabetic patients, who present with severe dry (foot) skin only. Please also be aware that Flexitol™ contains lanolin so may cause allergic issues in some patients. In addition, any application of ointment/cream to feet can increase the risk of slips and falls. There has also been an MHRA drug alert to highlight the increased risk of injury, due to fire, with paraffin based emollients on clothing. Patients should be reminded of these safety issues every time a cream or ointment is prescribed. Potential alternative Readily available to purchase OTC Indicated for Not indicated for Diabetics with severe dry (foot) skin Cosmetic Purposes Non-diabetics Supporting resources Diabetic Patient Letter Non-Diabetic Patient Letter Drug Alert: Fire hazard with paraffin-based skin products (November 2007) Return to start

IMPORTANT INFORMATION ABOUT YOUR MEDICATION Dear (Insert patients name) As a practice are always striving to provide the best possible care for our patients, and part of this process, involves the continual review of the medicines we prescribe. We are currently reviewing the usage of Flexitol products in line with current Walsall CCG guidelines. We have identified that you have been prescribed this treatment. However there is limited evidence available for these products. As you understand, foot care is a vital part of the management of your diabetes, including your annual diabetic foot checks. We would therefore be grateful if you could discuss this with your nurse/GP at your next appointment, so that the need for this item can be reviewed. Should you wish to continue using the Flexitol brand, it is available to purchase from the pharmacy. Please speak to your pharmacist about this. Should you have any queries or concerns, please do not hesitate to contact the practice. Yours sincerely Please not that all patient letters are available as a separate Word document. If you do not have access to these, please contact your practice pharmacist. Return to flexitol guidance Return to start

IMPORTANT INFORMATION ABOUT YOUR MEDICATION Dear (Insert patients name) As a practice, we are always striving to provide the best possible care for our patients, and part of this process, involves the continual review of the medicines we prescribe. We are currently reviewing the usage of Flexitol products in line with current Walsall CCG guidelines. We have identified that you have been prescribed this treatment. There is limited evidence available for these products, and it is not making the best use of NHS resources when used for cosmetic purposes. Should you wish to continue using this product, it is available to purchase from any pharmacy. Please speak to your pharmacist for further advice. As a practice we will no longer be prescribing this treatment and will be removing it from a repeat prescription. Should you have any queries or concerns, please do not hesitate to contact the practice. Yours sincerely Please not that all patient letters are available as a separate Word document. If you do not have access to these, please contact your practice pharmacist. Return to flexitol guidance Return to start

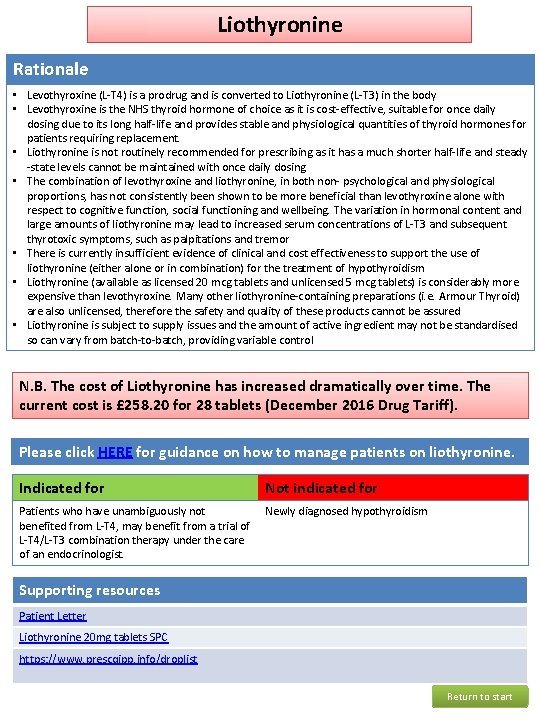
Liothyronine Rationale • Levothyroxine (L-T 4) is a prodrug and is converted to Liothyronine (L-T 3) in the body • Levothyroxine is the NHS thyroid hormone of choice as it is cost-effective, suitable for once daily dosing due to its long half-life and provides stable and physiological quantities of thyroid hormones for patients requiring replacement. • Liothyronine is not routinely recommended for prescribing as it has a much shorter half-life and steady -state levels cannot be maintained with once daily dosing • The combination of levothyroxine and liothyronine, in both non- psychological and physiological proportions, has not consistently been shown to be more beneficial than levothyroxine alone with respect to cognitive function, social functioning and wellbeing. The variation in hormonal content and large amounts of liothyronine may lead to increased serum concentrations of L-T 3 and subsequent thyrotoxic symptoms, such as palpitations and tremor • There is currently insufficient evidence of clinical and cost effectiveness to support the use of liothyronine (either alone or in combination) for the treatment of hypothyroidism • Liothyronine (available as licensed 20 mcg tablets and unlicensed 5 mcg tablets) is considerably more expensive than levothyroxine. Many other liothyronine-containing preparations (i. e. Armour Thyroid) are also unlicensed, therefore the safety and quality of these products cannot be assured • Liothyronine is subject to supply issues and the amount of active ingredient may not be standardised so can vary from batch-to-batch, providing variable control N. B. The cost of Liothyronine has increased dramatically over time. The current cost is £ 258. 20 for 28 tablets (December 2016 Drug Tariff). Please click HERE for guidance on how to manage patients on liothyronine. Indicated for Not indicated for Patients who have unambiguously not benefited from L-T 4, may benefit from a trial of L-T 4/L-T 3 combination therapy under the care of an endocrinologist. Newly diagnosed hypothyroidism Supporting resources Patient Letter Liothyronine 20 mg tablets SPC https: //www. prescqipp. info/droplist Return to start

The review process for patients on Liothyronine: • Consider optimising dose of levothyroxine • Consider seeking specialist advice if considering stopping liothyronine and increasing levothyroxine dose • Patients should have repeat TFTs 1 -2 months after switching to determine the appropriateness of their new dose. Patients under the care of a specialist endocrinologist should be referred back to consider suitability of switching in partnership. The BNF states that 20– 25 mcg of Liothyronine is equivalent to 100 mcg of levothyroxine. Return to liothyronine guidance Return to start

IMPORTANT INFORMATION ABOUT YOUR MEDICATION Dear (Insert patients name) This practice constantly reviews repeat prescriptions to make sure that our patients get the most effective treatment, which provides good value for the NHS without affecting their quality of care. We are currently reviewing the medicines we use to treat an underactive thyroid, particularly the medicine liothyronine (which is sometimes used with another thyroid hormone called levothyroxine). Please make an appointment to see your GP/practice nurse [delete where appropriate] to discuss your medication further. If you have any questions about this change please contact your GP/Nurse/Pharmacist. Yours sincerely Please not that all patient letters are available as a separate Word document. If you do not have access to these, please contact your practice pharmacist. Return to liothyronine guidance Return to start

Cough and Cold remedies Rationale Limited clinical value for these treatments – cough mixtures, aromatic inhalations, decongestants, sore throat lozenges etc. E. g. pholcodine linctus; simple linctus; nasal decongestants- xylometazoline; ephedrine; systemic decongestants- pseudoephedrine; aromatic inhalations- Menthol & eucalyptus inhalation. Both the Royal College of General Practitioners and the GMC state that GPs must encourage patients to self-care. Over the counter products are suitable for most patients’ in-line with their product licenses. Many products are not prescribable on NHS prescriptions as set out in Part XVIIIA of the Drug Tariff. Potential alternative Purchase as over-the-counter products. Promotion of self-care Indicated for Not indicated for N/A Supporting resources PATIENT LEAFLETS Cough in Adults information leaflet http: //www. selfcareforum. org/wp-content/uploads/2013/04/7 -Cough. pdf Sore Throat information leaflet http: //www. selfcareforum. org/wp-content/uploads/2013/04/10 -Sore-Throat. pdf The common cold in adults information leaflet http: //www. selfcareforum. org/wp-content/uploads/2013/03/20131030 -SCF-Fact. Sheet-No-12 -Common-Cold-v 1 -final. pdf Caring for children with coughs leaflet http: //www. bristol. ac. uk/medialibrary/sites/primaryhealthcare/documents/target/caring-for-children-with-coughleaflet-print-ready. pdf TARGET leaflets by RCGP. http: //www. rcgp. org. uk/clinical-and-research/toolkits/target-antibioticstoolkit/patient-information-leaflets. aspx EVIDENCE NICE CKS: Common Cold http: //cks. nice. org. uk/common-cold#!scenario Return to start

RCGP – TARGET LEAFLET The full PDF and word versions are available by clicking HERE. This also provides access to the leaflet in other languages. Return to start

Doxazosin Modified Release (MR) Rationale • Doxazosin has a long half-life of 22 hours making it suitable for once daily dosing Doxazosin immediate release and MR forms are available and they are both administered once daily and so a MR version of doxazosin offers no advantage in terms of patient compliance. • There are no apparent differences in the type of adverse events reported in studies and the SPC. The MR version may have a slightly lower overall incidence of adverse effects, but most adverse effects are mild. • Check and ensure that prescribing of doxazosin for hypertension is in line with NICE Clinical Guideline 127, Aug 2011 or prescribing of doxazosin for BPH is in line with NICE Clinical Guideline 97 May 2010. Commence new patients requiring doxazosin on the immediate release tablet version of doxazosin. • Review patients on doxazosin MR for suitability for switching to immediate release doxazosin tablets. • Switch patients to immediate release doxazosin tablets where it is clinically suitable to switch them. Indicated for Not indicated for Doxazosin is licensed for the treatment of hypertension and benign prostatic hypertrophy (BPH) Any other indications. Potential alternative Doxazosin immediate release. Click HERE for guidance on converting from modified to immediate release formulations. Supporting resources 1. UKMI Q&A 101. 3 How should conversion between doxazosin formulations be carried out? May 2009. www. nelm. nhs. uk 2. Summary of Product Characteristics. Cardura. Pfizer Limited. Accessed 24/10/2016 available at www. medicines. org. uk 3. Summary of Product Characteristics. Cardura XL. Pfizer Limited. Accessed on 24/10/2016 https: //www. medicines. org. uk/emc/medicine/4484 4. NICE Clinical Guideline 127, August 2011. Hypertension: Clinical management of primary hypertension in adults. Accessed on 9/2/12 and available at www. nice. org. uk 5. https: //www. prescqipp. info/droplist 5. NICE Clinical Guideline 97, May 2010. The management of lower urinary tract symptoms in men. Accessed 14/2/12 and available at www. nice. org. uk Return to start

Converting from modified to immediate release doxazosin Switching from modified-release to standard preparation In the absence of any firm recommendations from the manufacturers of modifiedrelease doxazosin, there are two possible strategies to convert patients from modified release to standard doxazosin and both scenarios require follow up monitoring of blood pressure and patient tolerability: 1. Give half the dose of modified-release doxazosin as standard doxazosin, i. e. 4 mg XL switched to 2 mg standard. There may be some patients who may require a higher dose and subsequent dose titration may be required. Or 2. Give the same dose as modified-release doxazosin but there may be some patients who suffer orthostatic hypotension and need a lower dose and subsequent dose titration may be required. The alternative is to comply with the licensed dosing recommendations and initiate therapy at 1 mg daily, increasing at weekly/fortnightly intervals. Whichever dose is chosen, it is important to monitor blood pressure (about 4 weeks after the switch) and patient symptoms closely and adjust the dose if necessary. More switching information on Presc. QIPP. Return to Doxazosin guidance Return to start

IMPORTANT INFORMATION ABOUT YOUR MEDICATION Dear (Insert patients name) This practice constantly reviews repeat prescriptions to make sure that our patients get the most effective treatment, which provides good value for the NHS without affecting their quality of care. We are currently reviewing the medicines we use to treat hypertension or BPH, particularly the medicine doxazosin mr Please make an appointment to see your GP/practice nurse [delete where appropriate] to discuss your medication further. If you have any questions about this change please contact your GP/Nurse/Pharmacist. Yours sincerely Please not that all patient letters are available as a separate Word document. If you do not have access to these, please contact your practice pharmacist. Return to doxazosin guidance Return to start

Omega 3 Rationale • The NICE guideline on lipid modification recommends that people with or at high risk of CVD should be advised to consume at least 2 portions of fish per week, including a portion of oily fish. However, it advises that omega-3 fatty acid compounds should not be offered for primary or secondary prevention of CVD, alone or in combination with a statin, including in people with CKD or type 1 or type 2 diabetes. Moreover, the guideline recommends that healthcare professionals should tell people that there is no evidence that omega‑ 3 fatty acid compounds help to prevent CVD. In addition, the NICE guideline on secondary prevention of myocardial infarction (MI) recommends that healthcare professionals should not offer or advise people who have had an MI to use omega‑ 3 fatty acid capsules or omega‑ 3 fatty acid supplemented foods to prevent another MI. Potential alternative Patients should be advised to increase their dietary intake of omega-3 fatty acids. • For hypertriglyceridemia, if used in conjunction with statins, consider a trial of statin in conjunction with increased dietary intake. If used in conjunction with diet, consider a switch to a fibrate. • For post-MI use, omega-3 fatty acids should be stopped. Ensure patients are receiving optimal secondary prevention medication. Patients who have had an MI should be advised to consume two to four portions of oily fish or other dietary sources per week. • For schizophrenia (unlicensed), ensure response is monitored carefully. Consideration to stopping therapy, in conjunction with specialist opinion, should be undertaken if no improvement is seen after 3 months use. • For all other indications, use is unlicensed and evidence of benefit is weak or non-existent. Patients should be advised that supplements are available to purchase if required or to increase their dietary intake if they wish. NICE indications Not indicated for No indications Schizophrenia Myocardial Infarction (Primary prevention) Multiple Sclerosis Familial Hypercholesterolaemia Supporting resources https: //www. prescqipp. info/-omega-3 -fatty-acids/send/85 -omega-3 -fatty-acids/787 -bulletin-47 omega-3 -fatty-acids http: //www. nhs. uk/Livewell/superfoods/Pages/is-oily-fish-a-superfood. aspx Omega 3 Food Fact sheet. BDA (2014). https: //www. bda. uk. com/foodfacts/omega 3. pdf Patient Letter Return to start

IMPORTANT INFORMATION ABOUT YOUR MEDICATION Dear (Insert patients name) We regularly review the medicines we prescribe to check that we are using the most effective and good value medicines. You are currently taking omega-3 fatty acid compounds and we have decided to stop prescribing of these products. There is insufficient evidence to show that these products have beneficial effects for your condition. It is important, however, to maintain enough fish oils or other forms of omega-3 fatty acids in your diet. The attached leaflet provides lots of information on dietary sources of omega-3 fatty acid compounds. If you are taking this product because you have had a heart attack (myocardial infarction), the National Institute for Health and Care Excellence (NICE) recommend that you eat at least two to four portions of oily fish per week. We do not expect you to notice any difference when your prescription is stopped. However, should you have any questions about this medicine change, please ask to speak to your doctor, practice nurse or community pharmacist. Please finish the remaining omega-3 fatty acid compounds tablets that you have at home. We will add any additional medicines if we feel these are appropriate when we review you in 2 months time Yours sincerely Please not that all patient letters are available as a separate Word document. If you do not have access to these, please contact your practice pharmacist. Return to Omega-3 guidance Return to start

Eflornithine 11. 5% cream (Vaniqa) Rationale The treatment of hirsuitism is a cosmetic procedure which is a low priority for funding by Walsall CCG. If hirsuitism is mild and does not significantly interfere with the woman’s quality of life, consider no additional treatment. Hirsutism is not usually associated with any significant medical abnormality. Eflornithine 11. 5% cream offers very little benefit for the management of facial hirsuitism in women. There is limited evidence for efficacy and patient satisfaction with eflornithine. Self-funded cosmetic treatments for reduction in hair growth or hair removal (e. g. shaving, threading, plucking, laser treatment, electrolysis) should be the primary options for the majority of women with hirsuitism. If additional treatment is required, consider offering co-cyprindiol. Potential alternative • • Self-funded cosmetic treatments for reduction in hair growth or hair removal Consider offering co-cyprindiol. Indicated for Not indicated for Co-cyprindiol- Treatment of moderate to severe hirsuitism, in women of reproductive age. N/A Supporting resources Patient Letter – cessation of prescribing Patient Letter – Invite for review SPC for co-cyprindiol Clinical Knowledge Summaries- Hirsutism https: //www. prescqipp. info/droplist Return to start

IMPORTANT INFORMATION ABOUT YOUR MEDICATION Dear (Insert patients name) We are currently reviewing the usage of eflornithine 11. 5% cream, also known as Vaniqa® which has been prescribed for excessive hair growth (hirsuitism). This treatment is considered a cosmetic treatment and experts recommend selfcare for the majority of women instead of being prescribed treatment. As a practice we will no longer be prescribing this treatment and have removed it from your repeat prescription. This will help us to use our limited NHS resources to prioritise treatment where it is most needed. You can try one of the following methods of hair removal as self-funded treatment if you still wish to treat your hirsuitism: • Shaving, plucking, waxing or threading • Laser hair removal • Electrolysis. You can discuss the best form of treatment for you with a beauty therapist. If you have found the eflornithine to be effective and wish to continue to use it you will need to obtain a private prescription for its supply and self-fund this treatment. Yours sincerely Please not that all patient letters are available as a separate Word document. If you do not have access to these, please contact your practice pharmacist. Return to Vaniqa guidance Return to start

IMPORTANT INFORMATION ABOUT YOUR MEDICATION Dear (Insert patients name) We are currently reviewing the usage of eflornithine 11. 5% cream, also known as Vaniqa® which has been prescribed for excessive hair growth (hirsuitism). We have undertaken an audit of our prescribing of eflornithine cream and have identified that you have not had your prescription reviewed recently. Please contact the surgery on the number below to arrange an appointment for a review of your medicines. Surgery telephone number 01922 xxxxxx Yours sincerely Please not that all patient letters are available as a separate Word document. If you do not have access to these, please contact your practice pharmacist. Return to Vaniqa guidance Return to start

Colecalciferol (Vitamin D 3) Rationale – ALWAYS PRESCRIBE BY LICENSED BRAND Many clinicians prescribe vitamin D 3 generically as colecalciferol, in a wide range of strengths and formulations. Prescriptions written generically are often fulfilled with unlicensed preparations e. g. Pro. D 3, Colevit D 3, Solgar Vitamin D 3 Osteocaps D 3, Aciferol D 3, Sunvit D 3, Hux D 3. Recently, there has been an increase of licensed low and high dose vitamin D 3 products available on the UK market. Licensed products should always be considered first line over unlicensed alternatives. See GMC Guidance on prescribing unlicensed medicines. Recommend self care wherever possible for prophylaxis of vitamin D deficiency for those who are at-risk. Potential alternatives (PRESCRIBE BY LICENSED BRAND) Desunin Fultium D 3 Aviticol Thorens Invita D 3 Strivit D 3 Healthy Start Vitamins – Available for FREE Healthy Start children’s vitamin drops (For children from 6 months old up until their 4 th birthday). - Vitamin A (233 micrograms), vitamin C (20 mg) vitamin D (7. 5 mcg) - Children who are having 500 ml or more of formula a day do not need Healthy Start vitamins. - The vitamins are suitable for vegetarians and free from milk, egg, gluten, soya and peanut residues. - Healthy Start women’s vitamin tablets (For pregnant women, women with a child under 12 months). - The daily dose is one tablet, which contains: Folic acid (400 mcg), vitamin C (70 mg) vitamin D (10 mcg). - They are suitable for vegetarians and free from wheat, fish, egg, salt. No colours, flavours or preservatives. No gluten containing ingredients. For further information click HERE For the nearest stockists click HERE Unlicensed preparations to avoid Colecalciferol (written generically) Pro D 3 Colevit D 3 Solgar Vitamin D 3 Sunvit D 3 Osteocaps D 3 Aciferol D 3 Hux D 3 Indicated for – For further information refer to individual product SPC (HERE) Supporting resources Patient letter BDA Food Fact Sheet: Vitamin D Presc. QIPP bulletin 120 (April 2016)- Vitamin D prescribed as colecalciferol (D 3) or ergocalciferol (D 2) (SPOT-List) Return to start

Walsall CCG Vitamin D formulary products Desunin tablets (V) 4000 iu 800 iu Fultium-D 3 capsules 800 iu 3, 200 iu 20, 000 iu Fultium drops 3 drops = 200 iu In. Vita D 3 (V) 2, 400 iu drops 1 drop = 67 iu 6 drops = 400 iu 25, 000 iu oral solution 50, 000 iu oral solution Aviticol capsule 20, 000 iu Alfacalcidol capsules Calcitriol capsules Thorens (V) 10, 000 iu oral drops 1 drop = 200 iu 25, 000 iu oral solution Strivit D 3 capsules (V) - avoid in peanut or soya allergy 800 iu capsule Return to colecalciferol guidance Return to start

IMPORTANT INFORMATION REGARDING CHANGES TO YOUR REPEAT PRESCRIPTION Dear (Insert patients name) We are currently reviewing our patients prescribed a Vitamin D only preparation (i. e. name of unlicensed preparation) for previous Vitamin D deficiency or insufficiency (low Vitamin D levels). At the time we prescribed (name of unlicensed preparation) as this was widely available, it is however classed as a food supplement and therefore not a licensed preparation. We now have several licensed Vitamin D preparations available and it is best practice to always prescribe licensed preparations where available. Therefore your next prescription for: Colecalciferol capsules- name of unlicensed preparation HAS BEEN CHANGED TO Name of licensed preparation This change will be reflected on your next repeat prescription and will not have any adverse effect on your treatment. You can finish off any medication you currently have. Please do not hesitate to contact the GP, nurse or practice pharmacist at the surgery if you have any queries or would like any further information. Yours sincerely Please not that all patient letters are available as a separate Word document. If you do not have access to these, please contact your practice pharmacist. Return to colecalciferol guidance Return to start
![References Coeliac UK https www coeliac org ukhome Accessed14 11 2016 Electronic References • Coeliac UK https: //www. coeliac. org. uk/home/ Accessed]14. 11. 2016 • Electronic](https://slidetodoc.com/presentation_image_h2/56f0ae1e0d24d5c83fdc616c55e761e4/image-31.jpg)
References • Coeliac UK https: //www. coeliac. org. uk/home/ Accessed]14. 11. 2016 • Electronic Medicines Compendium. Summary of Product Characteristics. Liothyronine https: //www. medicines. org. uk/emc/medicine/24153 Accessed 14. 11. 16 • Electronic Medicines Compendium. Summary of Product Characteristics. Co-cyprindol. https: //www. medicines. org. uk/emc/medicine/32058 • GMC. Prescribing guidance: Prescribing unlicensed medicines • http: //www. gmc-uk. org/guidance/ethical_guidance/14327. asp • GMC. Outcomes for provisionally registered doctors with a licence to practise (The Trainee Doctor) July 2015 Available at http: //www. gmcuk. org/Outcomes_for_provisionally_registered_doctors_Jul_15. pdf_61407158. pdf Accessed 28. 11. 16 • NHSBSA Drug Tariff. December 2016. http: //www. drugtariff. nhsbsa. nhs. uk/#/00414430 DD_2/DD 00414138/Part VIIIA products L Accessed on 29. 11. 16 • NICE Clinical Knowledge Summaries. Hirsuitism. December 2014. https: //cks. nice. org. uk/hirsutism • NICE NG 20: Coeliac disease: recognition, assessment and management. Accessed 14. 11. 16 https: //www. nice. org. uk/guidance/ng 20 • NICE Clinical Guideline 127, August 2011. Hypertension: Clinical management of primary hypertension in adults. Accessed on 9/2/12 and available at www. nice. org. uk • NICE Clinical Guideline 97, May 2010. The management of lower urinary tract symptoms in men. Accessed 14/2/12 and available at www. nice. org. uk • Pres. QIPP DROP-List guidance • Royal Pharmaceutical Society of Great Britain. Medicines Optimisation: Helping patients to get the most of their medicines. May 2013. Available at http: //www. rpharms. com/promoting-pharmacy-pdfs/helping -patients-make-the-most-of-their-medicines. pdf • Summary of Product Characteristics. Cardura. Pfizer Limited. Accessed 24/10/2016 available at www. medicines. org. uk • Summary of Product Characteristics. Cardura XL. Pfizer Limited. Accessed on 24/10/2016 https: //www. medicines. org. uk/emc/medicine/4484 • The Association of UK Dieticians. BDS Food Fact Sheet. Omega 3. September 2014. Accessed 29. 11. 2016 • The Association of UK Dieticians. BDS Food Fact Sheet. Vitamin D. August 2016. Accessed 29. 11. 2016 • https: //www. bda. uk. com/foodfacts/omega 3. pdf • https: //www. bda. uk. com/foodfacts/Vitamin. D. pdf • The Royal College of General Practitioners. The RCGP Curriculum: Core Curriculum Statement, Being a General Practitioner. February 2016. Accessed 28. 11. 16 • The Self Care Forum • The University of Bristol • The Royal College of General Practitioners – TARGET antibiotics toolkit • UKMI Q&A 101. 3 How should conversion between doxazosin formulations be carried out? May 2009. www. nelm. nhs. uk Return to start
 Perfusion limited vs diffusion limited
Perfusion limited vs diffusion limited Bedside clinical guidelines partnership
Bedside clinical guidelines partnership Gerd clinical practice guidelines
Gerd clinical practice guidelines Clinical guidelines
Clinical guidelines Clinical guidelines
Clinical guidelines Easl guidelines decompensated cirrhosis
Easl guidelines decompensated cirrhosis Penciptaan nilai adalah
Penciptaan nilai adalah Midlands 2 west north
Midlands 2 west north Walsall junior youth league
Walsall junior youth league Walsall junior youth league
Walsall junior youth league Walsall council social services
Walsall council social services Childrens services walsall
Childrens services walsall Walsall ccg formulary
Walsall ccg formulary Walsall ccg formulary
Walsall ccg formulary Veterinary medicines directorate
Veterinary medicines directorate Glencoe health chapter 19 medicines and drugs
Glencoe health chapter 19 medicines and drugs George's marvellous medicine story
George's marvellous medicine story Medicines management programme
Medicines management programme Individual prescription method
Individual prescription method Niyog-niyogan benefits
Niyog-niyogan benefits European directorate for the quality of medicines
European directorate for the quality of medicines What steps did roosevelt take to solve trusts
What steps did roosevelt take to solve trusts Nhs dictionary of medicines and devices
Nhs dictionary of medicines and devices Medicines learning portal
Medicines learning portal Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines Abasagar
Abasagar Medicines complete
Medicines complete Cqc medicines management
Cqc medicines management Medicines information centre
Medicines information centre Medicinescomplete martindale
Medicinescomplete martindale Pharmaceutical schedule
Pharmaceutical schedule National medicines policy
National medicines policy