Respiratory System Diffusion Xinping Yue xyuelsuhsc edu Department


- Slides: 17

Respiratory System Diffusion Xinping Yue xyue@lsuhsc. edu Department of Physiology LSUHSC-NO


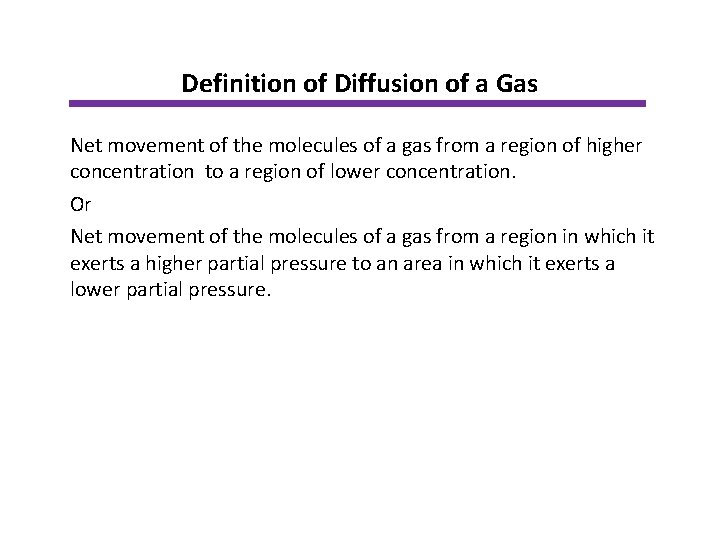
Definition of Diffusion of a Gas Net movement of the molecules of a gas from a region of higher concentration to a region of lower concentration. Or Net movement of the molecules of a gas from a region in which it exerts a higher partial pressure to an area in which it exerts a lower partial pressure.

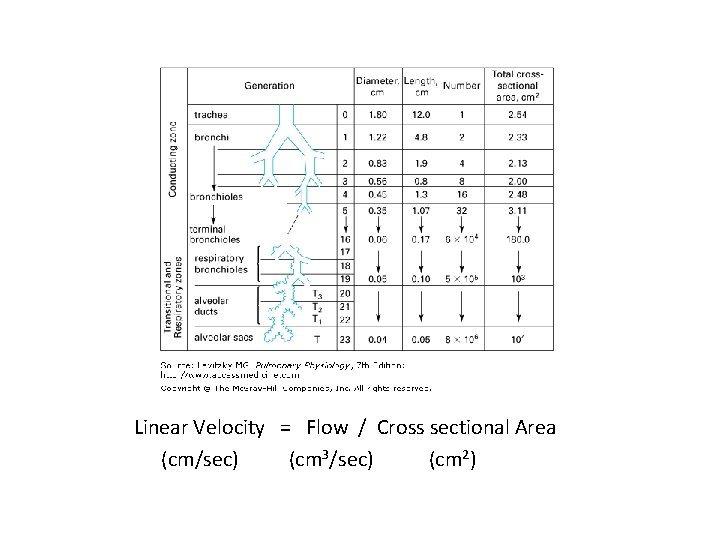
Linear Velocity = Flow / Cross sectional Area (cm/sec) (cm 3/sec) (cm 2)

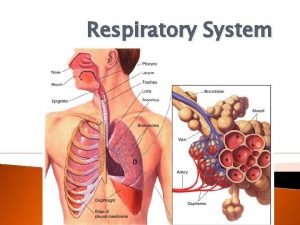
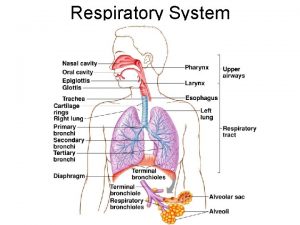
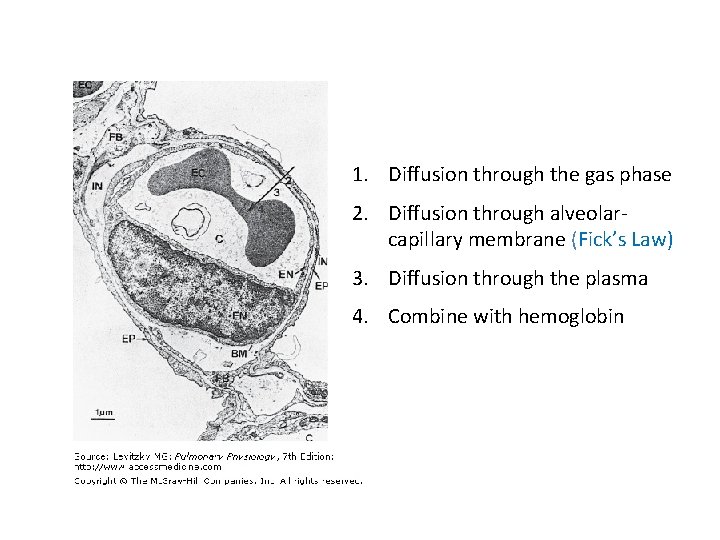
1. Diffusion through the gas phase 2. Diffusion through alveolarcapillary membrane (Fick’s Law) 3. Diffusion through the plasma 4. Combine with hemoglobin

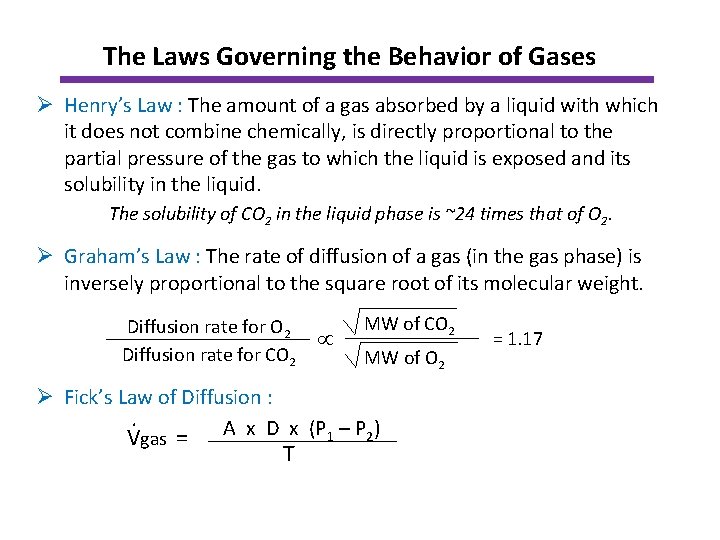
The Laws Governing the Behavior of Gases Ø Henry’s Law : The amount of a gas absorbed by a liquid with which it does not combine chemically, is directly proportional to the partial pressure of the gas to which the liquid is exposed and its solubility in the liquid. The solubility of CO 2 in the liquid phase is ~24 times that of O 2. Ø Graham’s Law : The rate of diffusion of a gas (in the gas phase) is inversely proportional to the square root of its molecular weight. Diffusion rate for O 2 Diffusion rate for CO 2 MW of O 2 Ø Fick’s Law of Diffusion : . Vgas = A x D x (P 1 – P 2) T = 1. 17

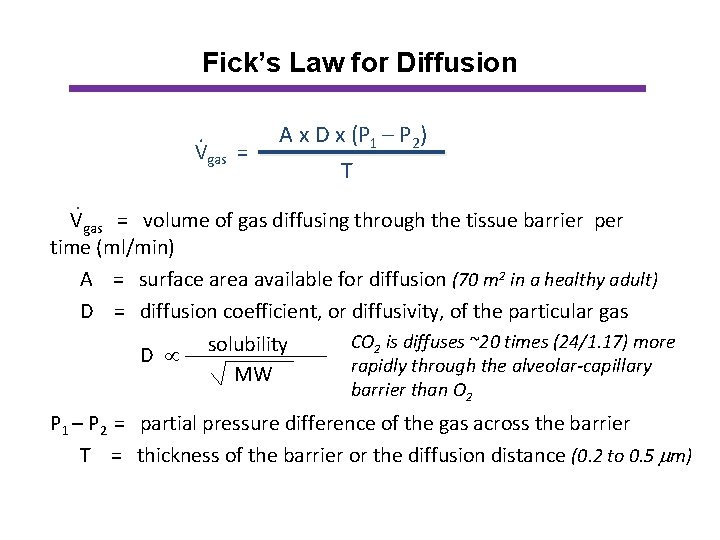
Fick’s Law for Diffusion. Vgas = A x D x (P 1 – P 2) T . Vgas = volume of gas diffusing through the tissue barrier per time (ml/min) A = surface area available for diffusion (70 m 2 in a healthy adult) D = diffusion coefficient, or diffusivity, of the particular gas CO 2 is diffuses ~20 times (24/1. 17) more solubility D rapidly through the alveolar-capillary MW barrier than O 2 P 1 – P 2 = partial pressure difference of the gas across the barrier T = thickness of the barrier or the diffusion distance (0. 2 to 0. 5 m)

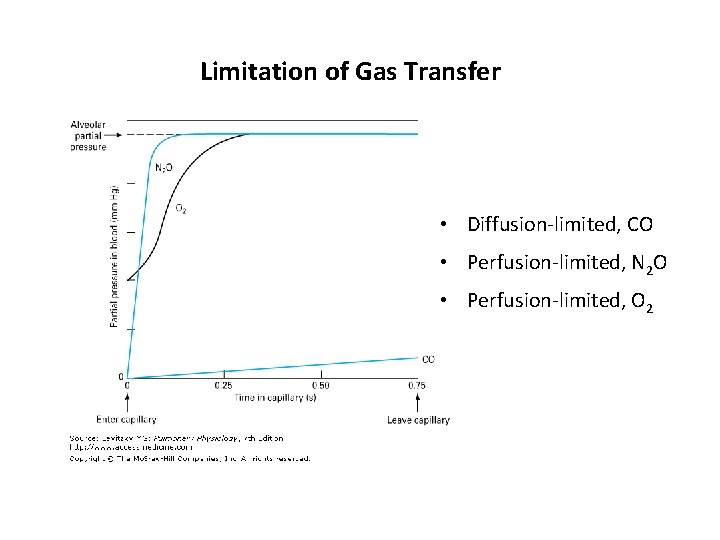
Limitation of Gas Transfer • Diffusion-limited, CO • Perfusion-limited, N 2 O • Perfusion-limited, O 2

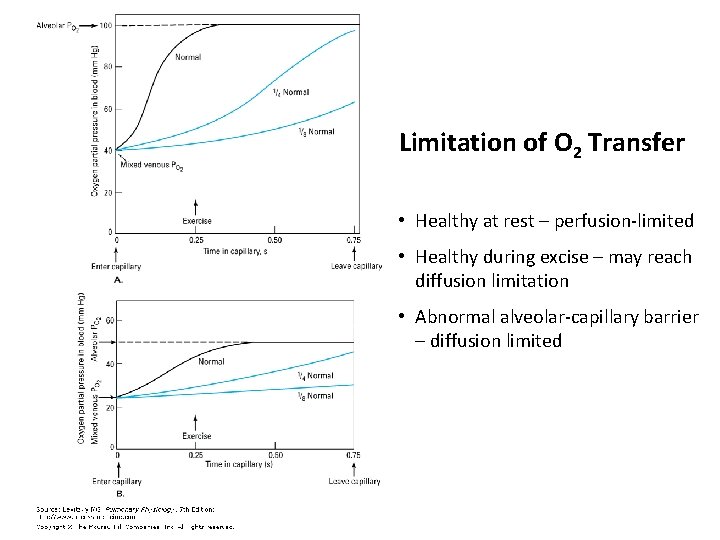
Limitation of O 2 Transfer • Healthy at rest – perfusion-limited • Healthy during excise – may reach diffusion limitation • Abnormal alveolar-capillary barrier – diffusion limited

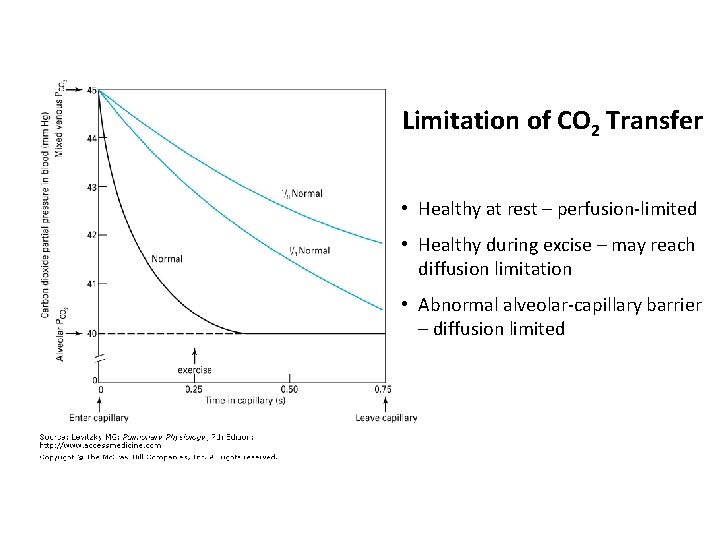
Limitation of CO 2 Transfer • Healthy at rest – perfusion-limited • Healthy during excise – may reach diffusion limitation • Abnormal alveolar-capillary barrier – diffusion limited

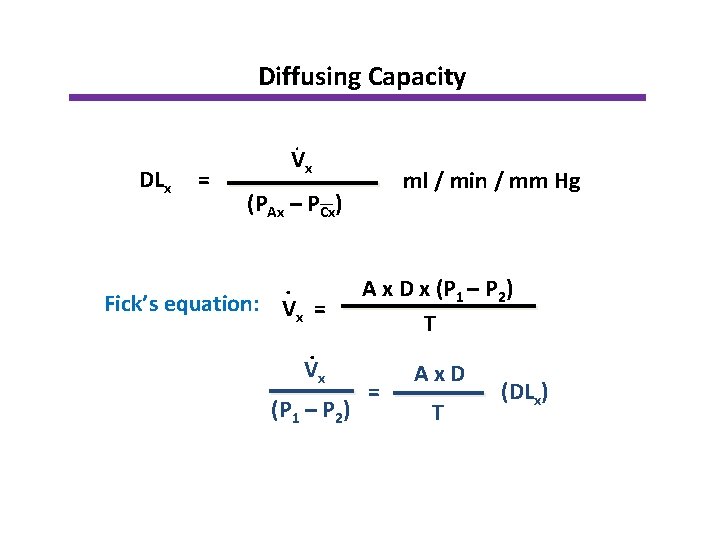
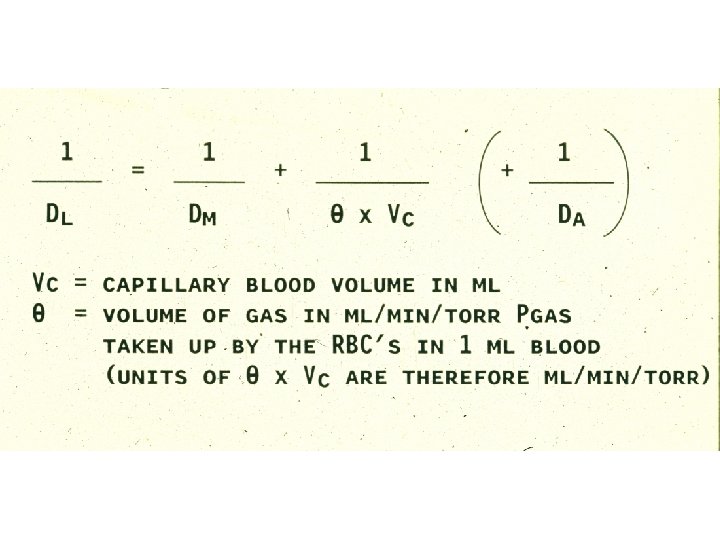
Diffusing Capacity DLx = . Vx ml / min / mm Hg (PAx – PCx) . Fick’s equation: Vx =. Vx (P 1 – P 2) A x D x (P 1 – P 2) T = Ax. D T (DLx)


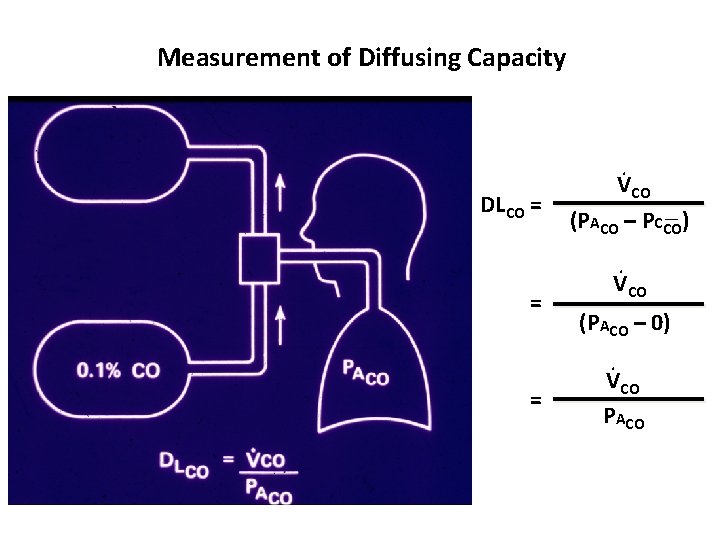
Measurement of Diffusing Capacity DLCO = = = . VCO (PACO – PCCO). VCO (PACO – 0). VCO PACO

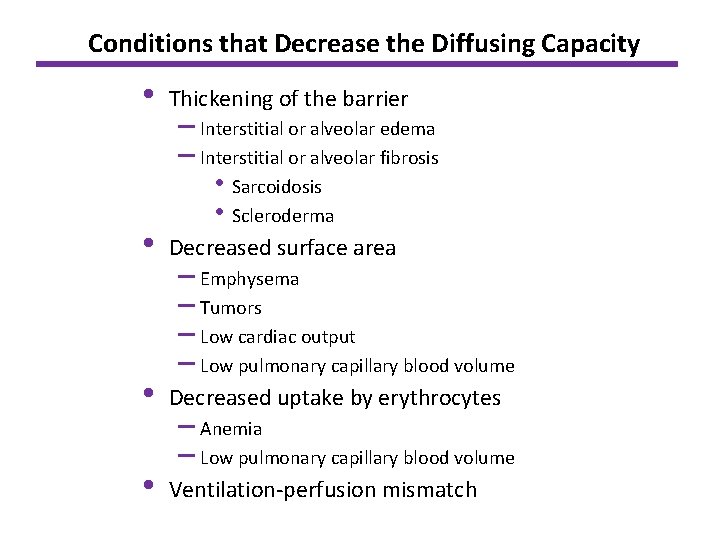
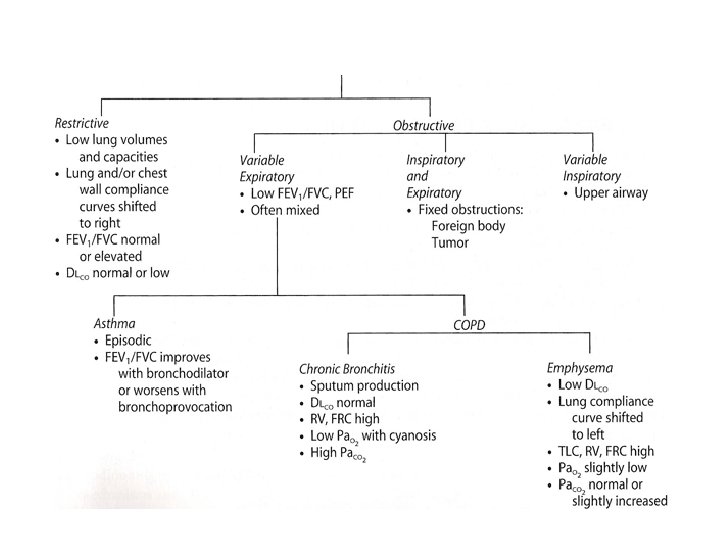
Conditions that Decrease the Diffusing Capacity • Thickening of the barrier • Decreased surface area • Decreased uptake by erythrocytes • Ventilation-perfusion mismatch – Interstitial or alveolar edema – Interstitial or alveolar fibrosis • Sarcoidosis • Scleroderma – Emphysema – Tumors – Low cardiac output – Low pulmonary capillary blood volume – Anemia – Low pulmonary capillary blood volume

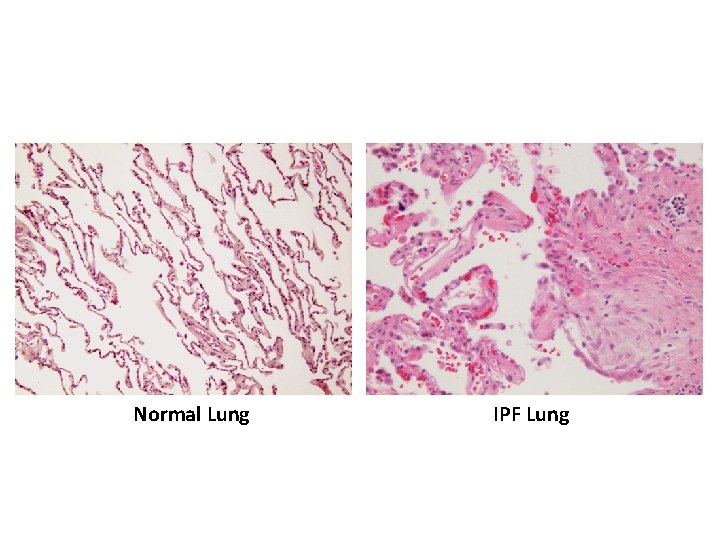
Normal Lung IPF Lung

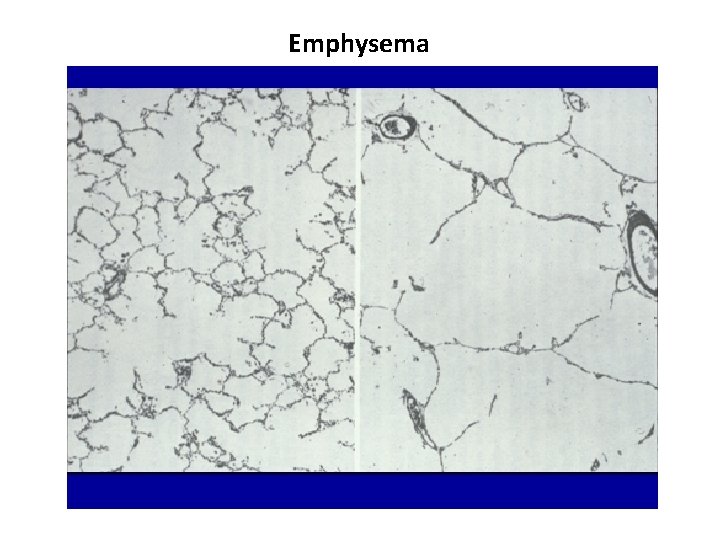
Emphysema

 Conducting zone respiratory
Conducting zone respiratory Simple diffusion
Simple diffusion Expansion diffusion definition
Expansion diffusion definition Diffusion of gases across respiratory membranes:
Diffusion of gases across respiratory membranes: Digestive respiratory and circulatory system
Digestive respiratory and circulatory system Yue minjun biography
Yue minjun biography Chuang qian ming yue guang li bai
Chuang qian ming yue guang li bai Ji yue ji hao meaning
Ji yue ji hao meaning Sarah yue
Sarah yue A question of dowry answers
A question of dowry answers Wenqi yue
Wenqi yue Nong li 7 yue
Nong li 7 yue Ivan kwong
Ivan kwong Yue kuo
Yue kuo Yue
Yue Tiny air sacs at the end of the bronchioles
Tiny air sacs at the end of the bronchioles Circulatory system and respiratory system work together
Circulatory system and respiratory system work together Edu.sharif.edu
Edu.sharif.edu