National Medicines Policy 2018 2022 a patientrelated approach







- Slides: 19

National Medicines Policy 2018– 2022 – a patient-related approach International perspective Marcin Czech Undersecretary of State at the Ministry of Health 2018

What is a national medicines policy? § it defines the aim, the scope and the lines for action § it presents and prioritises medium- and long-term aims set by the Government for pharmaceutical market participants, as well as identifies main strategies for their achievement § it represents the official position of the Government as a formal description of aspirations, objectives, decisions and commitments § it covers both public and private sectors and involves all major participants of the pharmaceutical market 2018

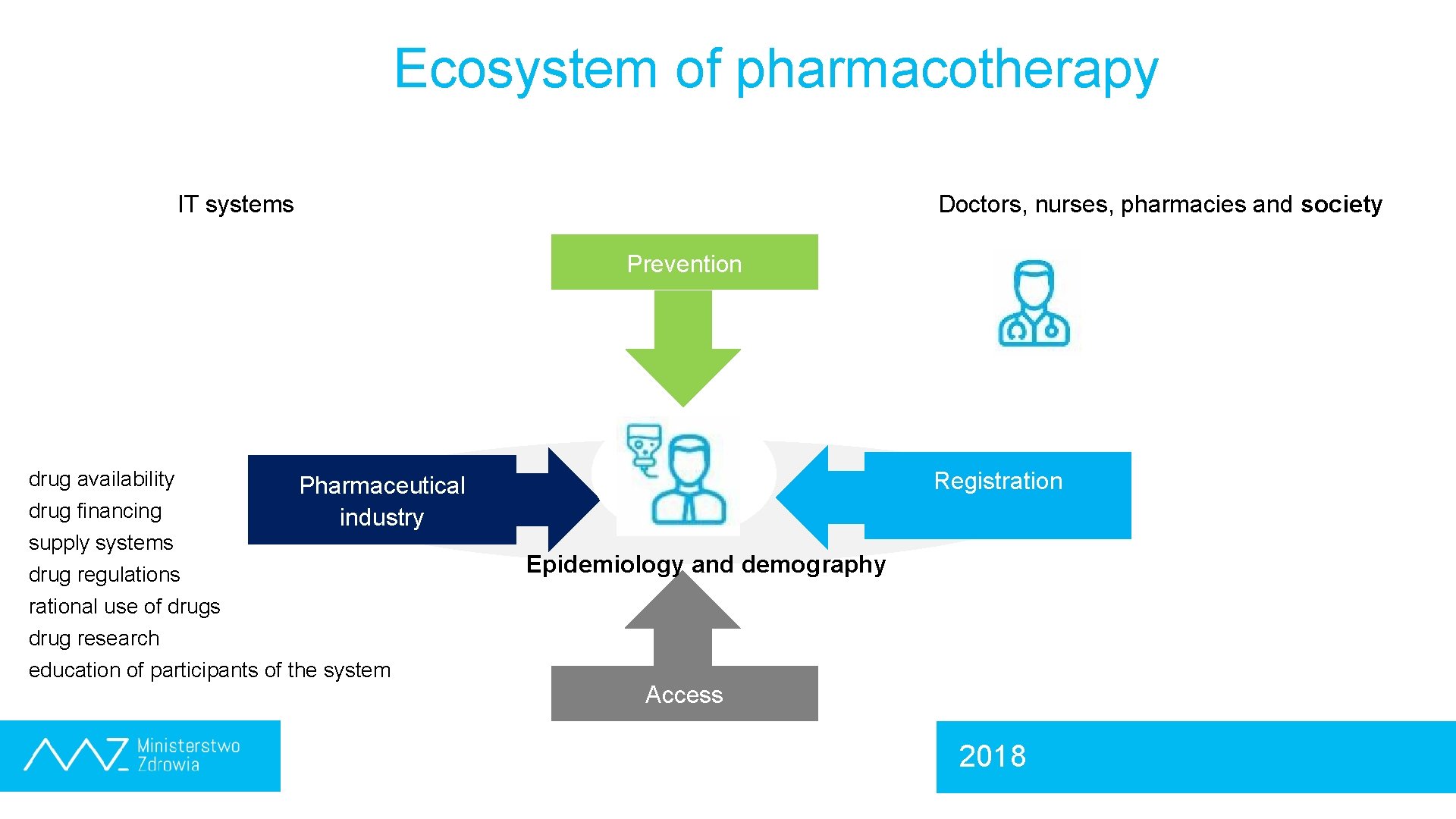
Ecosystem of pharmacotherapy IT systems Doctors, nurses, pharmacies and society Prevention drug availability drug financing supply systems Registration Pharmaceutical industry drug regulations Epidemiology and demography rational use of drugs drug research education of participants of the system Access 2018

Local vs. international § National Medicines Policy vs. Lancet guidelines § International initiatives in the National Medicines Policy document 2018

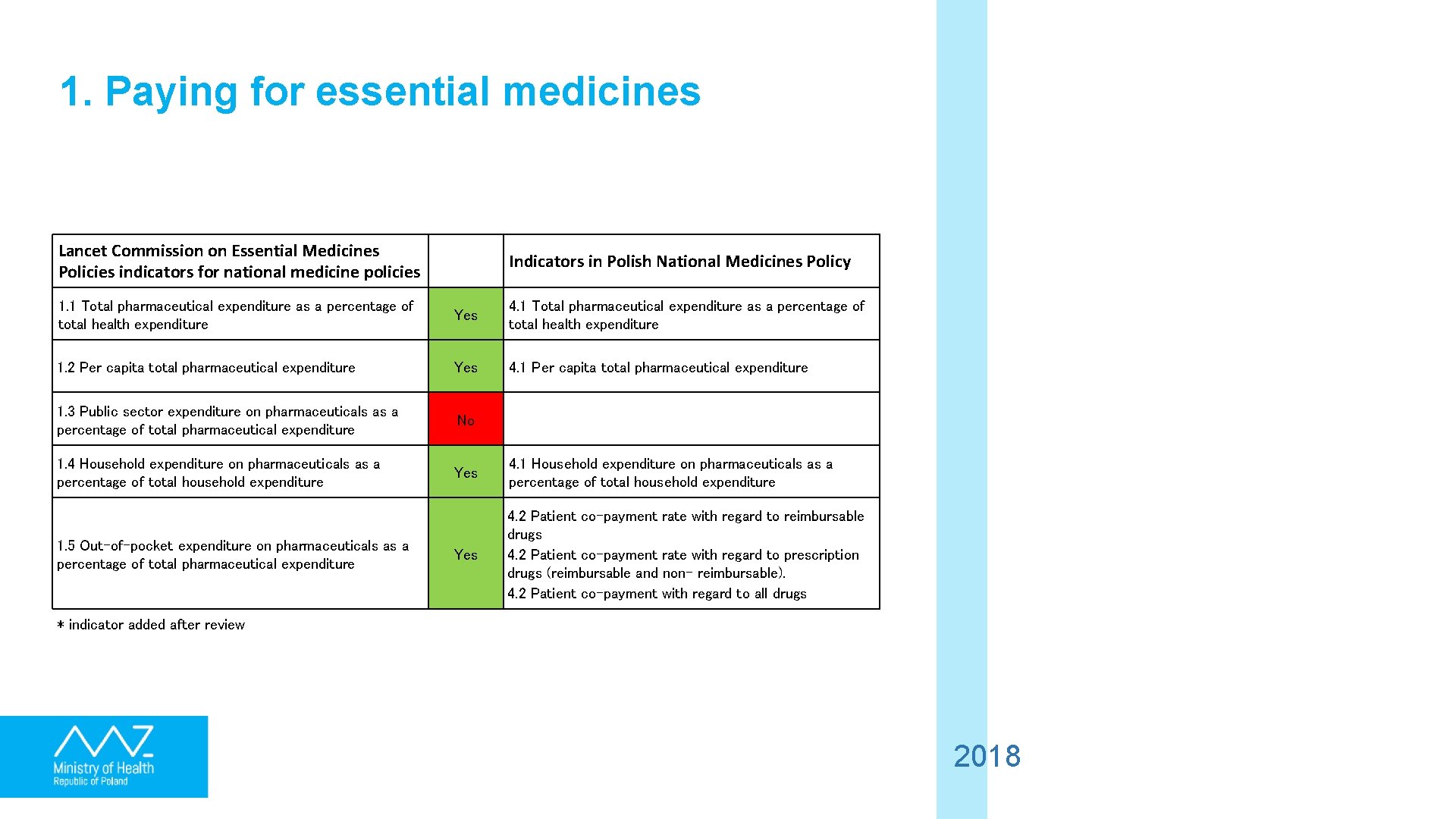
1. Paying for essential medicines Lancet Commission on Essential Medicines Policies indicators for national medicine policies Indicators in Polish National Medicines Policy 1. 1 Total pharmaceutical expenditure as a percentage of total health expenditure Yes 4. 1 Total pharmaceutical expenditure as a percentage of total health expenditure 1. 2 Per capita total pharmaceutical expenditure Yes 4. 1 Per capita total pharmaceutical expenditure 1. 3 Public sector expenditure on pharmaceuticals as a percentage of total pharmaceutical expenditure No 1. 4 Household expenditure on pharmaceuticals as a percentage of total household expenditure Yes 4. 1 Household expenditure on pharmaceuticals as a percentage of total household expenditure Yes 4. 2 Patient co-payment rate with regard to reimbursable drugs 4. 2 Patient co-payment rate with regard to prescription drugs (reimbursable and non- reimbursable). 4. 2 Patient co-payment with regard to all drugs 1. 5 Out-of-pocket expenditure on pharmaceuticals as a percentage of total pharmaceutical expenditure * indicator added after review 2018

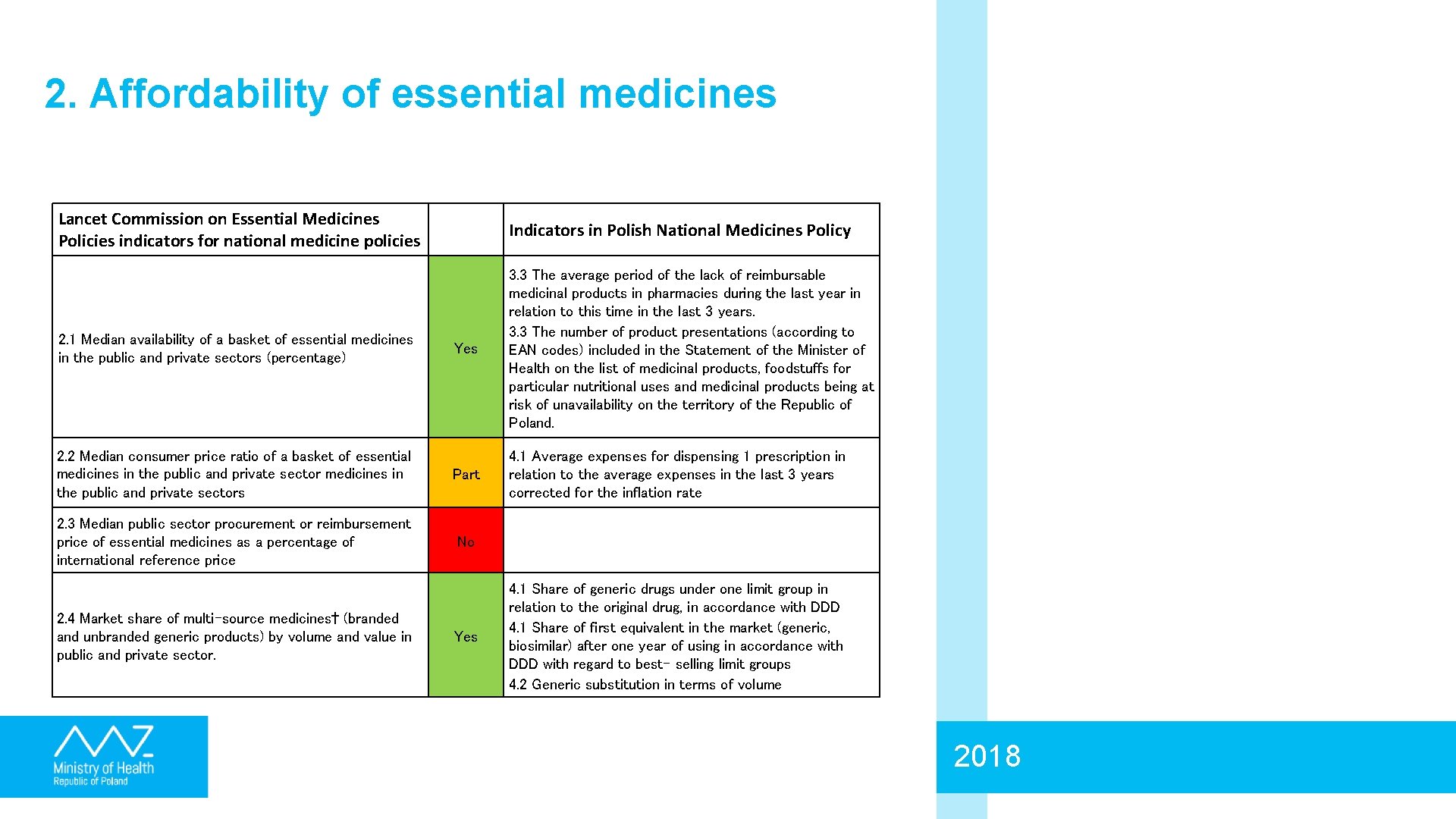
2. Affordability of essential medicines Lancet Commission on Essential Medicines Policies indicators for national medicine policies Indicators in Polish National Medicines Policy 2. 1 Median availability of a basket of essential medicines in the public and private sectors (percentage) Yes 3. 3 The average period of the lack of reimbursable medicinal products in pharmacies during the last year in relation to this time in the last 3 years. 3. 3 The number of product presentations (according to EAN codes) included in the Statement of the Minister of Health on the list of medicinal products, foodstuffs for particular nutritional uses and medicinal products being at risk of unavailability on the territory of the Republic of Poland. 2. 2 Median consumer price ratio of a basket of essential medicines in the public and private sectors Part 4. 1 Average expenses for dispensing 1 prescription in relation to the average expenses in the last 3 years corrected for the inflation rate 2. 3 Median public sector procurement or reimbursement price of essential medicines as a percentage of international reference price No 2. 4 Market share of multi-source medicines† (branded and unbranded generic products) by volume and value in public and private sector. Yes 4. 1 Share of generic drugs under one limit group in relation to the original drug, in accordance with DDD 4. 1 Share of first equivalent in the market (generic, biosimilar) after one year of using in accordance with DDD with regard to best- selling limit groups 4. 2 Generic substitution in terms of volume 2018

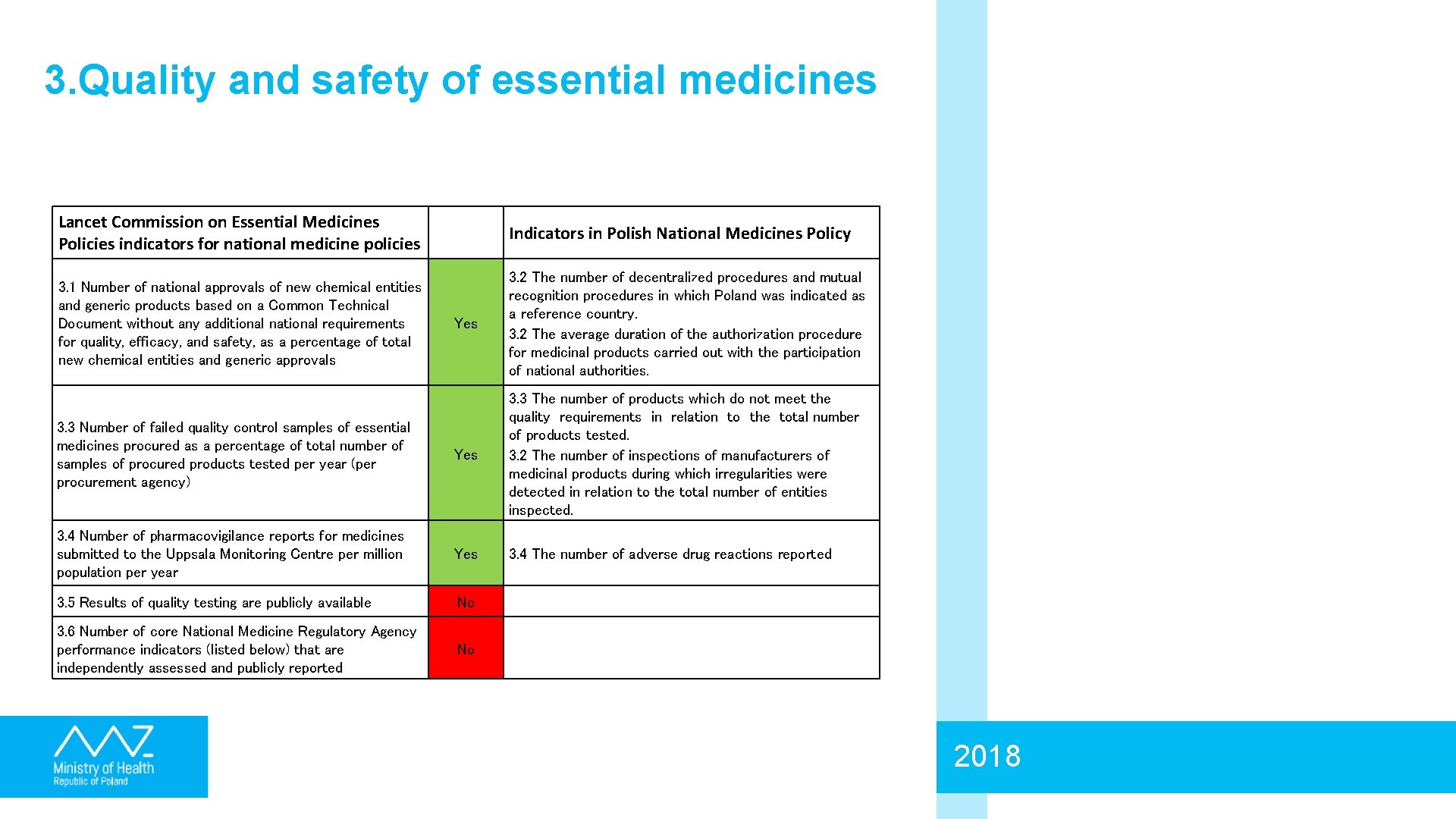
3. Quality and safety of essential medicines Lancet Commission on Essential Medicines Policies indicators for national medicine policies Indicators in Polish National Medicines Policy 3. 1 Number of national approvals of new chemical entities and generic products based on a Common Technical Document without any additional national requirements for quality, efficacy, and safety, as a percentage of total new chemical entities and generic approvals Yes 3. 2 The number of decentralized procedures and mutual recognition procedures in which Poland was indicated as a reference country. 3. 2 The average duration of the authorization procedure for medicinal products carried out with the participation of national authorities. 3. 3 Number of failed quality control samples of essential medicines procured as a percentage of total number of samples of procured products tested per year (per procurement agency) Yes 3. 3 The number of products which do not meet the quality requirements in relation to the total number of products tested. 3. 2 The number of inspections of manufacturers of medicinal products during which irregularities were detected in relation to the total number of entities inspected. 3. 4 Number of pharmacovigilance reports for medicines submitted to the Uppsala Monitoring Centre per million population per year Yes 3. 4 The number of adverse drug reactions reported 3. 5 Results of quality testing are publicly available No 3. 6 Number of core National Medicine Regulatory Agency performance indicators (listed below) that are independently assessed and publicly reported No 2018

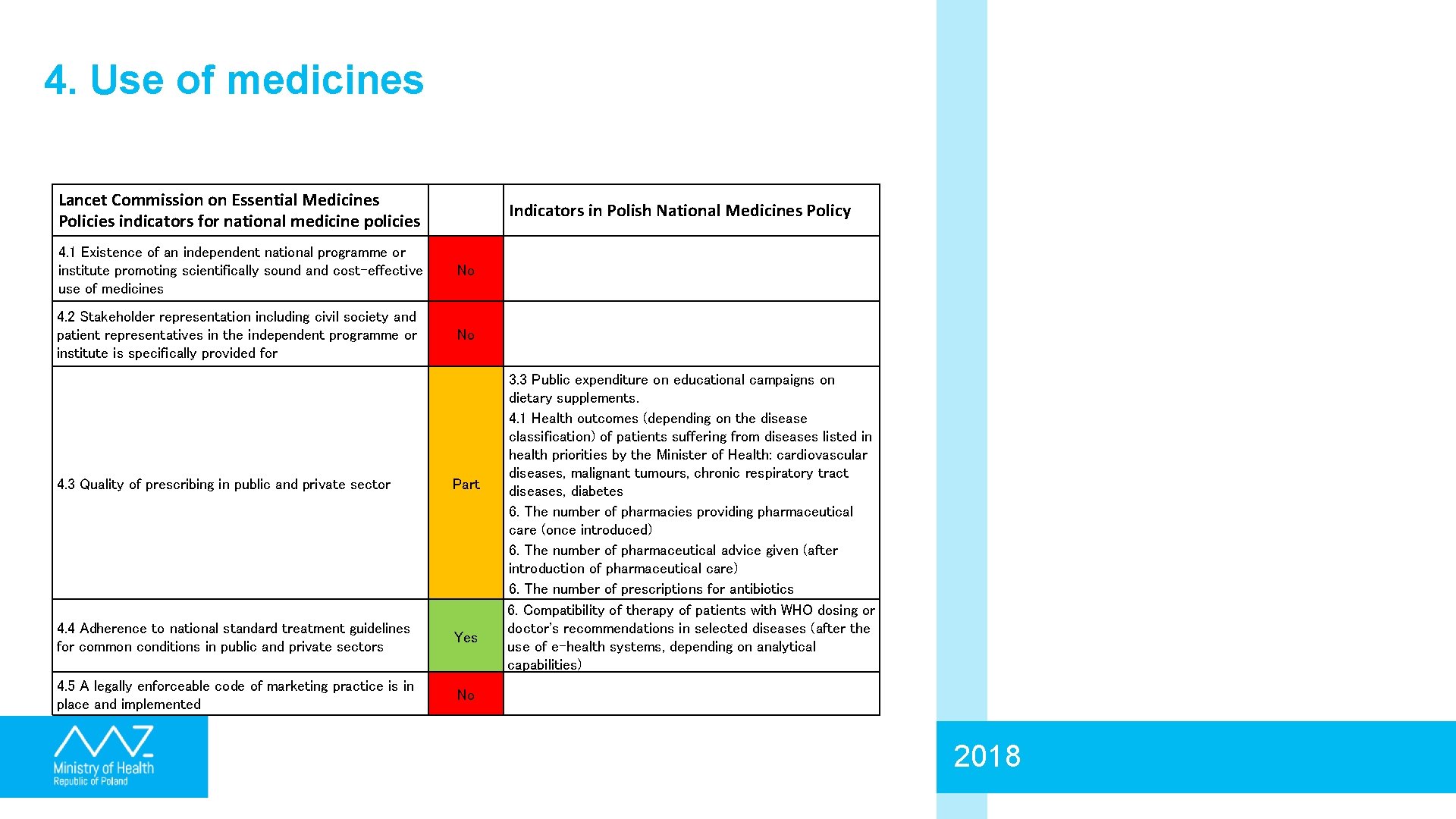
4. Use of medicines Lancet Commission on Essential Medicines Policies indicators for national medicine policies Indicators in Polish National Medicines Policy 4. 1 Existence of an independent national programme or institute promoting scientifically sound and cost-effective use of medicines No 4. 2 Stakeholder representation including civil society and patient representatives in the independent programme or institute is specifically provided for No 4. 3 Quality of prescribing in public and private sector Part 4. 4 Adherence to national standard treatment guidelines for common conditions in public and private sectors Yes 4. 5 A legally enforceable code of marketing practice is in place and implemented No 3. 3 Public expenditure on educational campaigns on dietary supplements. 4. 1 Health outcomes (depending on the disease classification) of patients suffering from diseases listed in health priorities by the Minister of Health: cardiovascular diseases, malignant tumours, chronic respiratory tract diseases, diabetes 6. The number of pharmacies providing pharmaceutical care (once introduced) 6. The number of pharmaceutical advice given (after introduction of pharmaceutical care) 6. The number of prescriptions for antibiotics 6. Compatibility of therapy of patients with WHO dosing or doctor's recommendations in selected diseases (after the use of e-health systems, depending on analytical capabilities) 2018

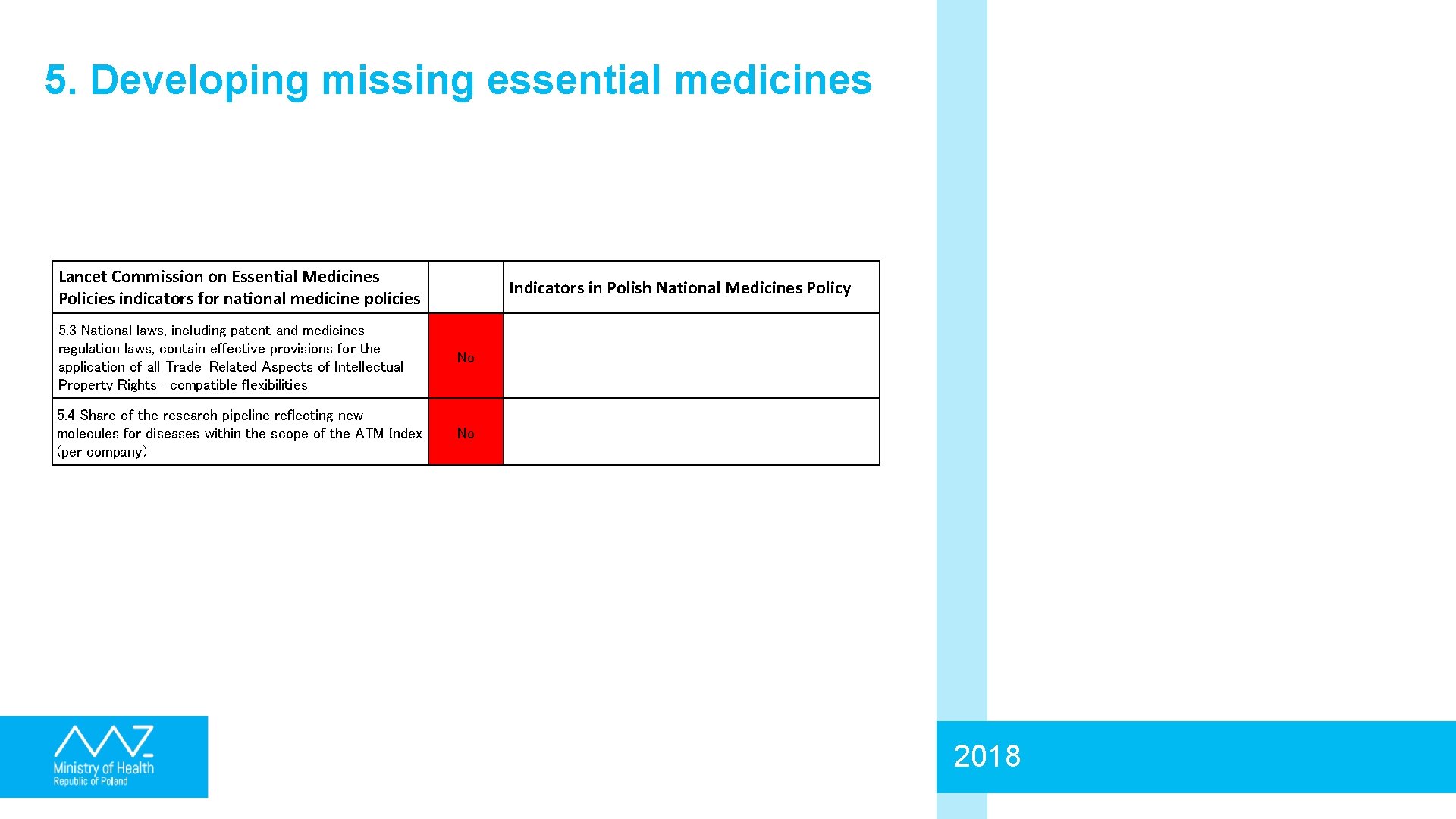
5. Developing missing essential medicines Lancet Commission on Essential Medicines Policies indicators for national medicine policies Indicators in Polish National Medicines Policy 5. 3 National laws, including patent and medicines regulation laws, contain effective provisions for the application of all Trade-Related Aspects of Intellectual Property Rights -compatible flexibilities No 5. 4 Share of the research pipeline reflecting new molecules for diseases within the scope of the ATM Index (per company) No 2018

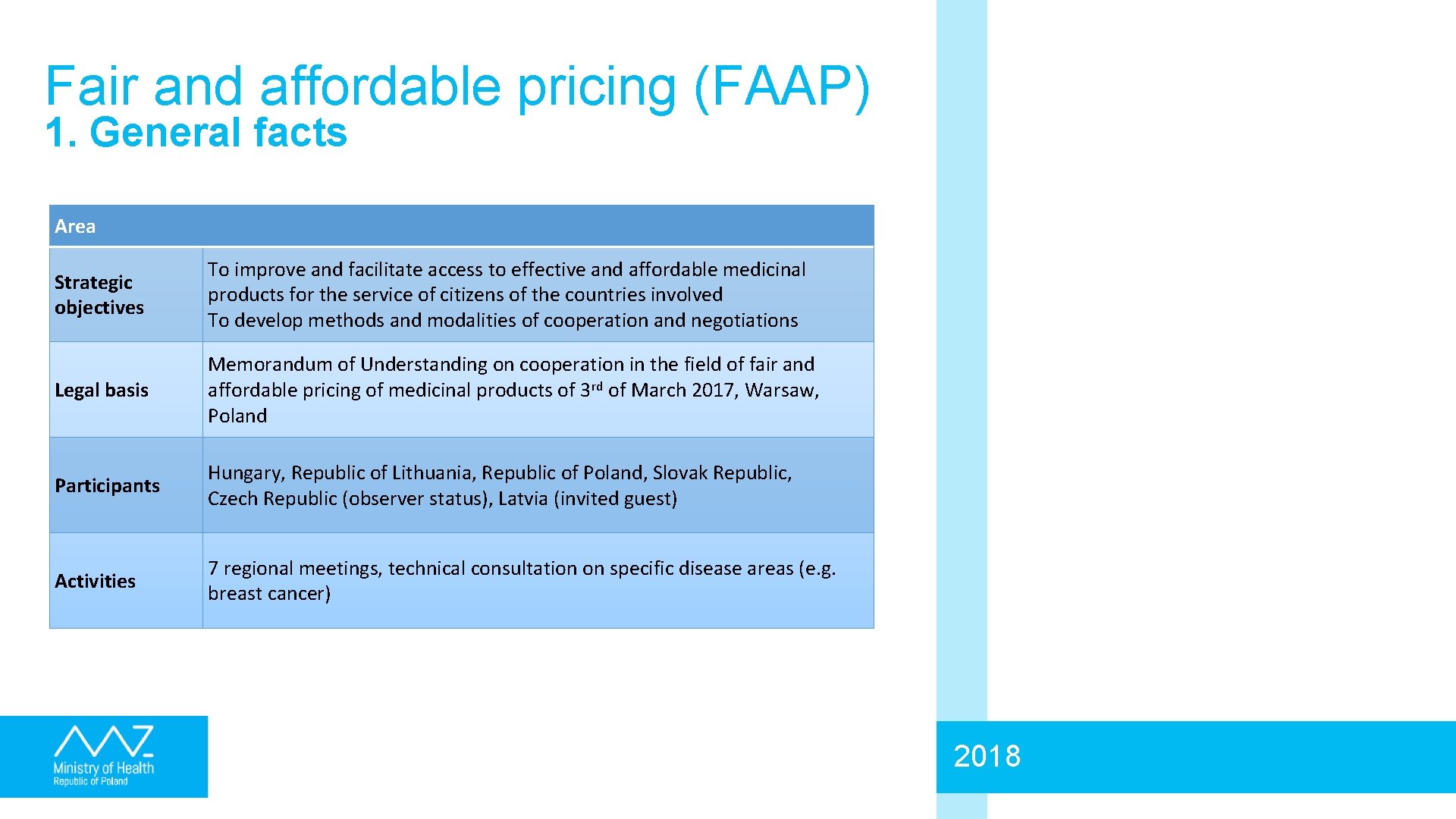
Fair and affordable pricing (FAAP) 1. General facts Area Strategic objectives To improve and facilitate access to effective and affordable medicinal products for the service of citizens of the countries involved To develop methods and modalities of cooperation and negotiations Legal basis Memorandum of Understanding on cooperation in the field of fair and affordable pricing of medicinal products of 3 rd of March 2017, Warsaw, Poland Participants Hungary, Republic of Lithuania, Republic of Poland, Slovak Republic, Czech Republic (observer status), Latvia (invited guest) Activities 7 regional meetings, technical consultation on specific disease areas (e. g. breast cancer) 2018

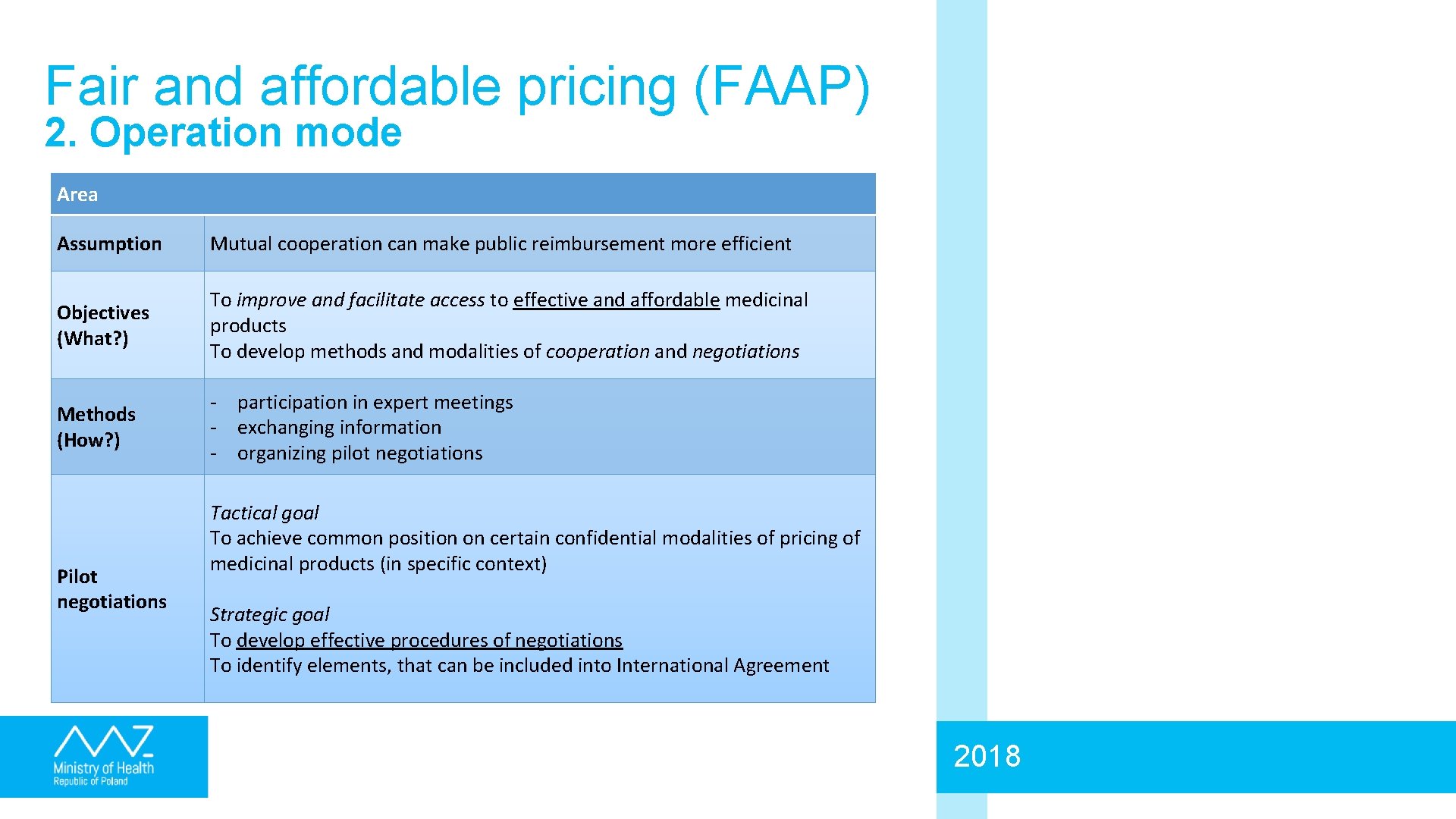
Fair and affordable pricing (FAAP) 2. Operation mode Area Assumption Mutual cooperation can make public reimbursement more efficient Objectives (What? ) To improve and facilitate access to effective and affordable medicinal products To develop methods and modalities of cooperation and negotiations Methods (How? ) - participation in expert meetings - exchanging information - organizing pilot negotiations Pilot negotiations Tactical goal To achieve common position on certain confidential modalities of pricing of medicinal products (in specific context) Strategic goal To develop effective procedures of negotiations To identify elements, that can be included into International Agreement 2018

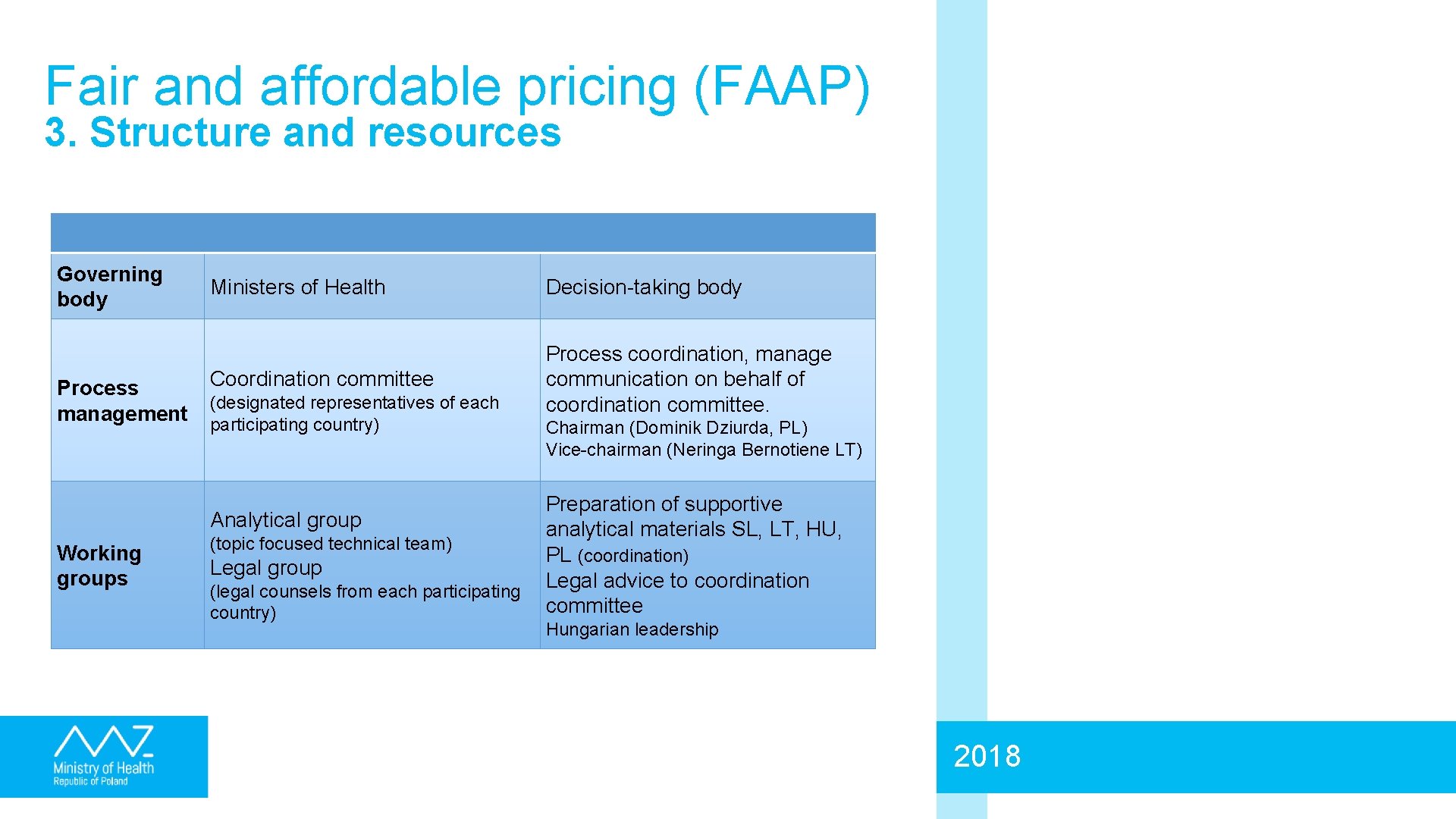
Fair and affordable pricing (FAAP) 3. Structure and resources Governing body Process management Ministers of Health Decision-taking body Coordination committee Process coordination, manage communication on behalf of coordination committee. (designated representatives of each participating country) Analytical group Working groups (topic focused technical team) Legal group (legal counsels from each participating country) Chairman (Dominik Dziurda, PL) Vice-chairman (Neringa Bernotiene LT) Preparation of supportive analytical materials SL, LT, HU, PL (coordination) Legal advice to coordination committee Hungarian leadership 2018

FAAP Initiative Key ideas/principles underlying the Initiative § Similar socioeconomics and health-related needs and challenges as well as geographic proximity as an underlying factors for close collaboration between Participants § Focus on facilitation of access to effective and affordable medicinal products for the service of citizens of the countries involved § FAAP initiative should be perceived as a complementary, missing element allowing better proactive preparation of local reimbursement systems for innovation (regional topic-specific reimbursement strategy) § Focus on certain modalities of pricing – any variables which specify the availability of medicinal products within national reimbursement system e. g. : clinical characteristics of patients, length of treatment, expected reimbursement mechanism, financial conditions etc. § The Memorandum which constitutes the legal basis for the FAAC initiative is not intended to create any legal obligations under domestic or international law. The ground of the initiative has been the intention of the Participants to effectively utilize the results of the Initiatives for the health benefits of its citizens 2018

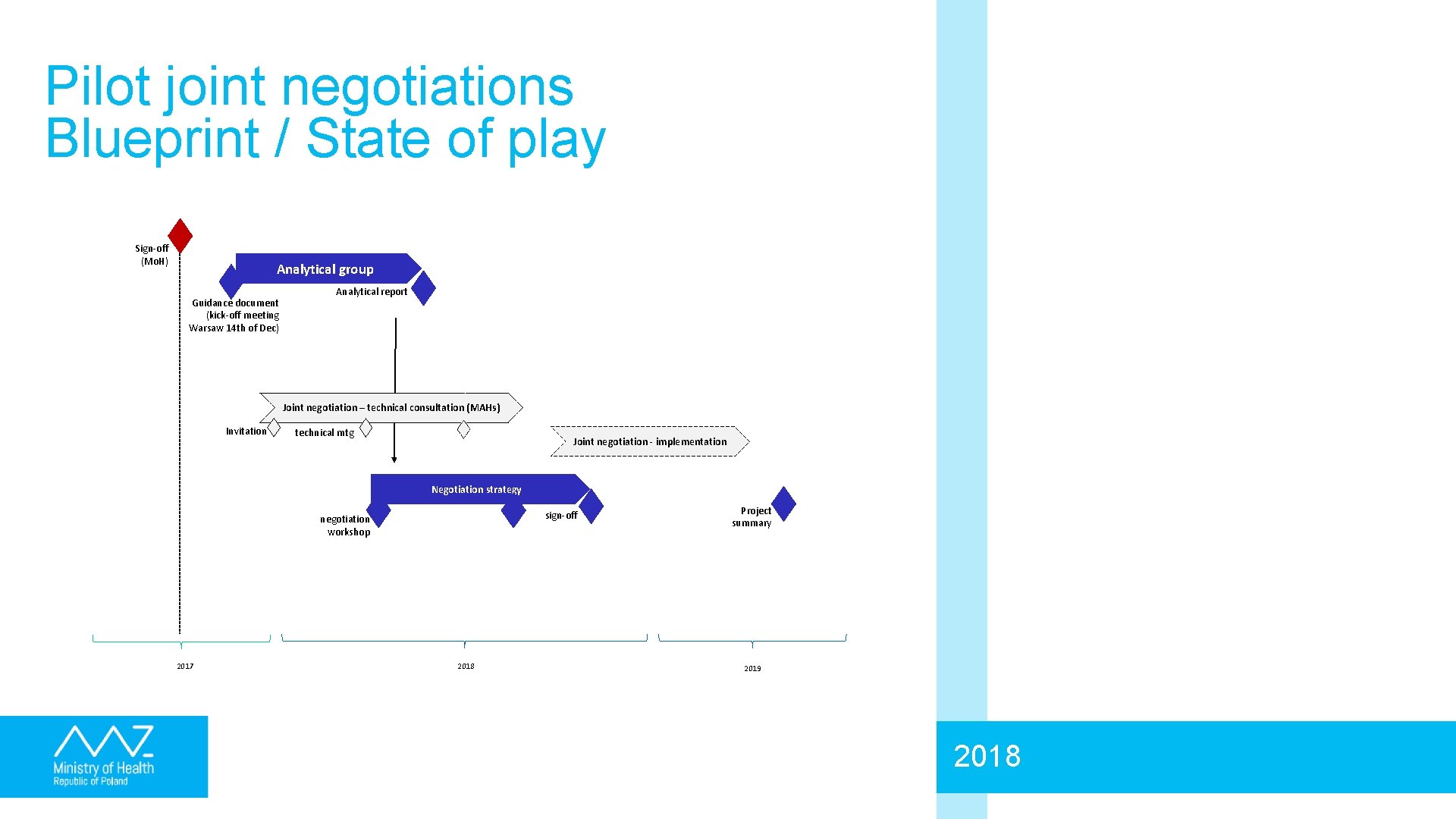
Pilot joint negotiations Blueprint / State of play Sign-off (Mo. H) Analytical group Guidance document (kick-off meeting Warsaw 14 th of Dec) Analytical report Joint negotiation – technical consultation (MAHs) Invitation technical mtg Joint negotiation - implementation Negotiation strategy sign-off negotiation workshop 2017 2018 Project summary 2019 2018

Fair and affordable pricing (FAAP) SUMMARY § The ultimate goal of FAP initiative is the development of methods and modalities of cooperation and negotiations with MAHs concerning pricing of certain medicinal technologies in order to improve and facilitate access to effective and affordable medicinal products on V 4+ level. § Above can be delivered through long-time cooperation focused on: ü Building an active institutional network within the region ü Exchange of expertise and experience in the field of reimbursement and pricing ü Common assessment of desired options for treatment management § Taking learning-by-doing / pilot approach the process assumes preparation of coherent regional negotiation strategy of development of public reimbursement system for specific disease area, which could stand as guidelines for national processes § Pilot process should allow to build recommendation on development an International Agreement concerning fair pricing 2018

2018

§ Aim: to ensure sustainable access to innovative medicine at affordable cost for our patients § Countries: Belgium, Netherlands, Luxemburg, Austria, Ireland ü Ireland joins Be. Ne. Lux. A initiative - June 2018, the Irish Minister for Health, Simon Harris signed an Agreement with his colleagues from Belgium, The Netherlands, Luxembourg and Austria to join the Beneluxa Initiative on Pharmaceutical Policy (Be. Ne. Lux. AI) § Recent achievement: Positive outcome of joint reimbursement negotiations on Spinraza (nusinersen) indicated for SMA 2018

Important initiative European Commission § HTA AND REGIONAL COOPERATION WORKSHOP ü September 2018 ü Organized by DG SANCO European Commission § Aim: to share experiences form the regional cooperation § International initiatives invited by EC & represented: ü Be. Ne. Lux. AIr ü La Valletta ü FINOSE ü Fair and affordable Pricing Initiative ü EUnet. HTA Joint Action 2018

Thank you for your attention 2018
 National medicines policy
National medicines policy Cog 2018 2022
Cog 2018 2022 Que letra continua m v t m j
Que letra continua m v t m j Veterinary medicines directorate
Veterinary medicines directorate Chapter 19 vocabulary glencoe health
Chapter 19 vocabulary glencoe health George's marvellous medicine story
George's marvellous medicine story Medicines management programme
Medicines management programme The drug basket method dispense medication to is used to
The drug basket method dispense medication to is used to Niyog niyogan uses and preparation
Niyog niyogan uses and preparation European directorate for the quality of medicines
European directorate for the quality of medicines What legislation helped solve dangerous food and medicines
What legislation helped solve dangerous food and medicines Nhs dictionary of medicines and devices
Nhs dictionary of medicines and devices Medicines learning portal
Medicines learning portal Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines European medicines agency
European medicines agency Medicines complete
Medicines complete Cqc medicines management
Cqc medicines management Medicines information centre
Medicines information centre Medicines complete
Medicines complete Pharmac schedule
Pharmac schedule