Unit 5 Counting particles that are too small











- Slides: 24

Unit 5! Counting particles that are too small to see…

Introduction • How many jelly beans/pennies/peanuts/etc do you think is in this jar? • How can you make an educated guess and win these sort of contests? • Think of physical properties ▫ Density ▫ Volume ▫ Mass

Jar of Pennies • How many pennies do you think is in this container? • If the container is 5 L • The density of a penny is approximately 8. 96 g/m. L • The approximate mass of a penny is ~3. 1 g

Dalton’s Theory • What can you say about the mass of 1 mole of hydrogen vs the mass of 1 mole of oxygen? • Dalton thought water was 1 part H and 1 part O to his death, why was he wrong? ▫ This leads us to theory of relative mass.

Theory of Relative Mass • What is Avogadro’s Hypothesis? ▫ At the same pressure and temperature, two containers will have the same number of particles ▫ We know standard temperature and pressure what is standard volume? ▫ Standard volume = 22. 4 L � 1 “mole” of an ideal gas will occupy 22. 4 L

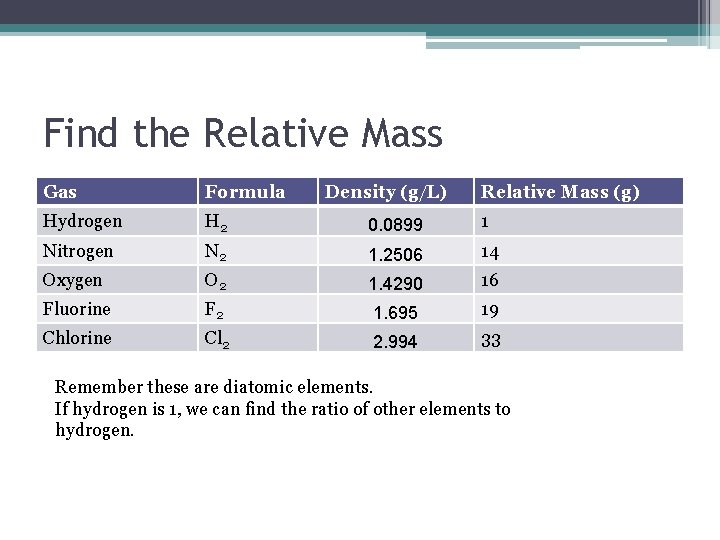
Find the Relative Mass Gas Formula Density (g/L) Relative Mass (g) Hydrogen H 2 0. 0899 1 Nitrogen N 2 1. 2506 14 Oxygen O 2 1. 4290 16 Fluorine F 2 1. 695 19 Chlorine Cl 2 2. 994 33 Remember these are diatomic elements. If hydrogen is 1, we can find the ratio of other elements to hydrogen.

Molar Mass – What is it? • The sum of the relative atomic masses of the elements present in a formula unit. • Example: What is the molar mass formula of hydrogen gas? ▫ Remember Hydrogen is a diatomic element, which means we have 2 hydrogen. ▫ Solution: Look at the periodic table and look at the atomic mass of hydrogen 1. 008 and multiply by 2 since we have two units hydrogen.

Molar Mass • • What is the molar mass of water? What is the molar mass of carbon dioxide? What is the molar mass of sodium chloride? What is the molar mass of Pb. Cl 2?

The Mole • No… Not this one • Or this one…

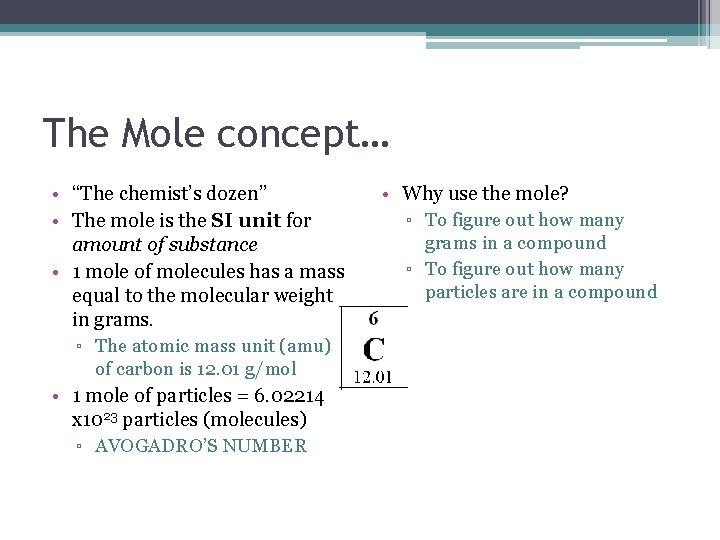
The Mole concept… • “The chemist’s dozen” • The mole is the SI unit for amount of substance • 1 mole of molecules has a mass equal to the molecular weight in grams. ▫ The atomic mass unit (amu) of carbon is 12. 01 g/mol • 1 mole of particles = 6. 02214 x 1023 particles (molecules) ▫ AVOGADRO’S NUMBER • Why use the mole? ▫ To figure out how many grams in a compound ▫ To figure out how many particles are in a compound

I’m confused, what is a mole? • Think of it this way: • A dozen elephants doesn’t have the same weight as a dozen rabbits, BUT in each case, you have a dozen animals. • A mole of oxygen gas has a different mass than a mole of hydrogen gas BUT in each case you have 6. 02 x 1023 molecules (if you have 1 mole of them)

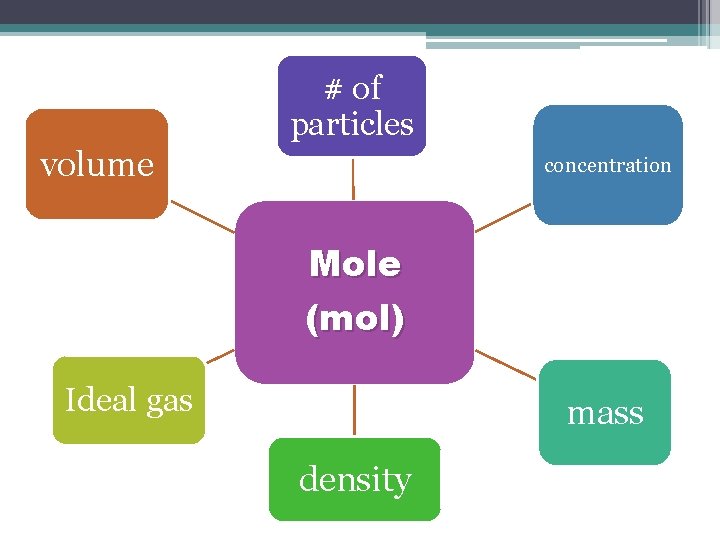
# of particles volume concentration Mole (mol) Ideal gas mass density

Size of a Mole n. To help you better visualize the enormous size of Avogadro's number, 6. 02 x 1023, consider the following analogies: 1. If we had a mole of rice grains, all the land area of the earth would be covered with rice to a depth of about 75 meters! 2. One mole of rice grains is more grain than the number of all grain grown since the beginning of time. 3. One mole of marshmallows (standard 1 in 3 size) would cover the United States to a depth of 650 miles.

Size of a Mole 4. If the Mount St. Helens eruption had released a mole of particles the size of sand grains, the entire state of Washington would have been buried to a depth equal to the height of a 10 -story building. 5. A mole of basketballs would just about fit perfectly into a ball bag the size of the earth.

Your turn! A. B. C. Assuming that each human being has 60 trillion body cells (6 x 1013) and that the earth's population is 7 billion (7 x 109): ▫ Calculate the total number of living human body cells on this planet. ▫ Is this number smaller or larger than a mole? Divide the larger value by the smaller to determine the relative size of the two values. One of the fastest supercomputers can perform about 12 teraflops (1 teraflop is 1012 calculations) every second. Determine how many seconds it would take this computer to do one moles worth of calculations. Convert this figure into years. If you started counting when you first learned how to count and then counted by ones, eight hours a day, 5 days a week for 50 weeks a year, you would be judged a 'good counter' if you could reach 4 billion by the time you retired at age 65. If every human on earth (about 7 x 109 people) were to count this way until retirement, what fraction of a mole would they count?

Mole: Molecules Conversions • Use the factor-label (dimensional analysis) method to convert substances from mass to particles (and vice versa) using moles. • Example: ▫ How many moles is in 17. 1 g of H 2 O? ▫ What mass of CO would you need to have 24. 6 moles of CO? ▫ How many molecules are in 10 moles of Na. Cl?

Review: Percent Composition by Mass • A 9. 86 g sample of Ca. S is found to contain 7. 35 g of calcium. What is the percent by mass of the calcium and sulfur in this compound?

Empirical vs. Molecular Formula • Empirical Formula ▫ Based on experimental data ▫ Simplest/lowest mole ratio of elements in a compound. ▫ CH 2 O • Molecular Formula ▫ Actual number of moles of elements in a compound. ▫ C 6 H 12 O 6 (n=6)

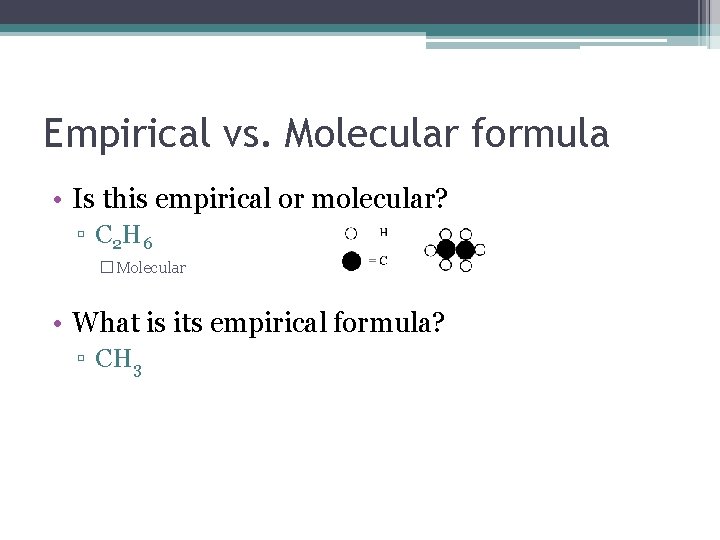
Empirical vs. Molecular formula • Is this empirical or molecular? ▫ C 2 H 6 � Molecular • What is its empirical formula? ▫ CH 3

Find the empirical & molecular formula • An unknown sample was tested to have 6. 9899 g C, 1. 1724 g H, and 9. 309 g O. ▫ Calculate the empirical formula for this sample. � 1 - Find percent composition of each element. � 2 - Find the simplest whole-number ratios between the elements. (USING MOLES) ▫ What is the molecular formula of this sample if the molar mass is 90. 079 g/mol? �n= molecular mass empirical formula �Multiply n to the empirical formula to find the molecular formula.




Percent by mass • Calculate the percent by mass of Carbon in the formula C 2 H 4 O 2 • Calculate the percent by mass of the other elements. • When calculating the empirical formula and the problem gives you percentages, assume 100 g sample.