Unit 3 Atomic Structure Atomic Mass Units amu



- Slides: 11

Unit 3: Atomic Structure Atomic Mass Units (amu) and Calculating Atomic Mass

Remember? ? ? • Mass number = protons + neutrons • Isotopes= atoms with different # of neutrons • Atomic masses occur with decimal points because they are an average of different isotopes

Atomic Mass Units • Defined as 1/12 the mass of a Carbon -12 atom. • The way that atomic mass is measured in the Periodic Table • Abbreviated “amu”

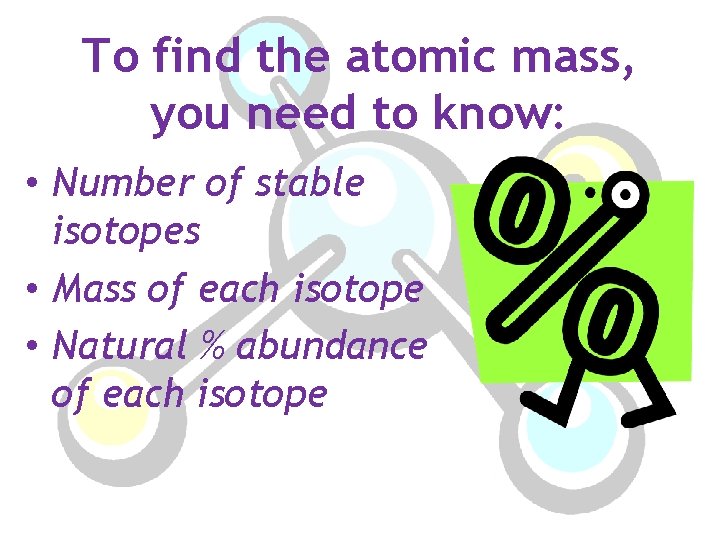
To find the atomic mass, you need to know: • Number of stable isotopes • Mass of each isotope • Natural % abundance of each isotope

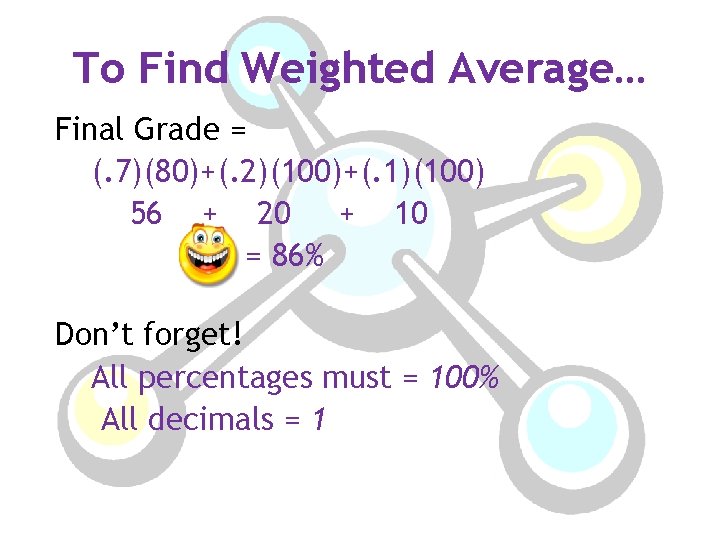
Real-World Application: Weighted Average Let’s just say that the weights that make up your grade are as follows: • Tests – 70% of grade • Quizzes – 20 % of grade • Homework – 10% of grade You earn the following grades: test – 80, quiz – 100, homework – 100

To Find Weighted Average… Final Grade = (. 7)(80)+(. 2)(100)+(. 1)(100) 56 + 20 + 10 = 86% Don’t forget! All percentages must = 100% All decimals = 1

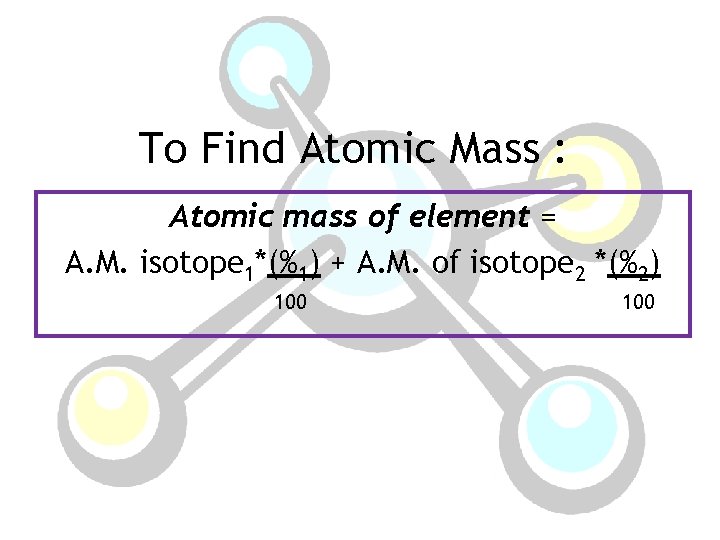
To Find Atomic Mass : Atomic mass of element = A. M. isotope 1*(%1) + A. M. of isotope 2 *(%2) 100

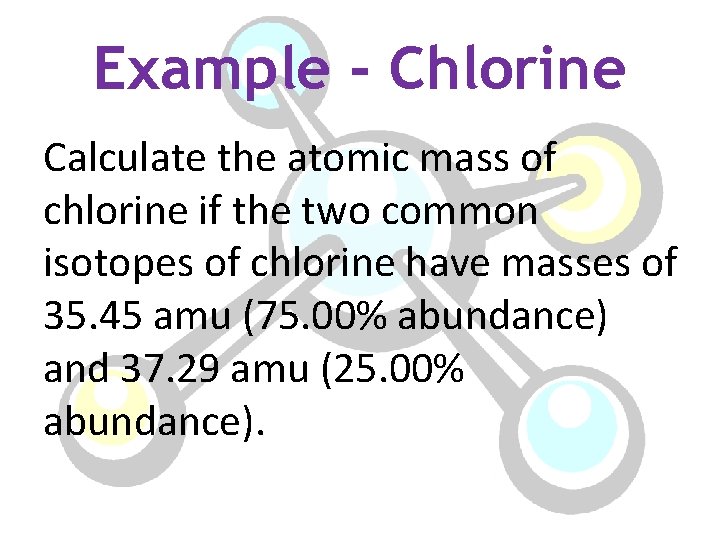
Example - Chlorine Calculate the atomic mass of chlorine if the two common isotopes of chlorine have masses of 35. 45 amu (75. 00% abundance) and 37. 29 amu (25. 00% abundance).

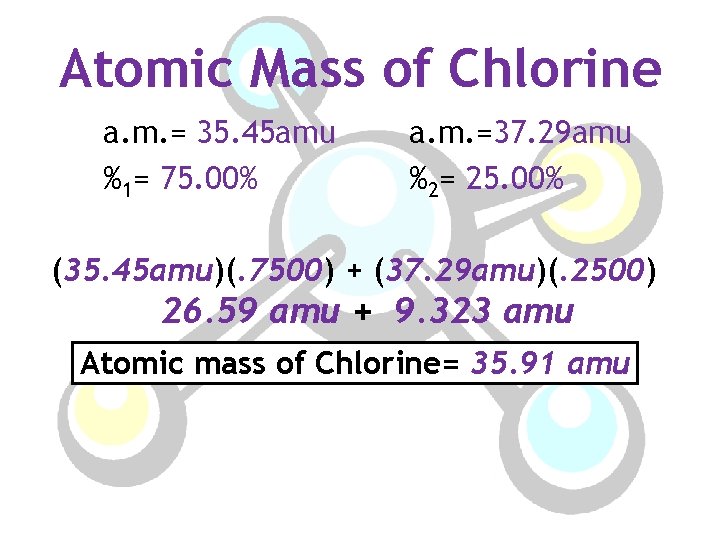
Atomic Mass of Chlorine a. m. = 35. 45 amu %1= 75. 00% a. m. =37. 29 amu %2= 25. 00% (35. 45 amu)(. 7500) + (37. 29 amu)(. 2500) 26. 59 amu + 9. 323 amu Atomic mass of Chlorine= 35. 91 amu

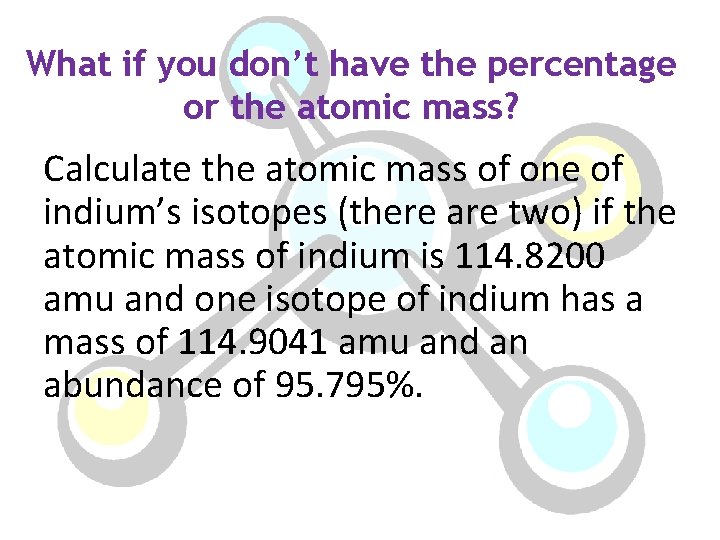
What if you don’t have the percentage or the atomic mass? Calculate the atomic mass of one of indium’s isotopes (there are two) if the atomic mass of indium is 114. 8200 amu and one isotope of indium has a mass of 114. 9041 amu and an abundance of 95. 795%.

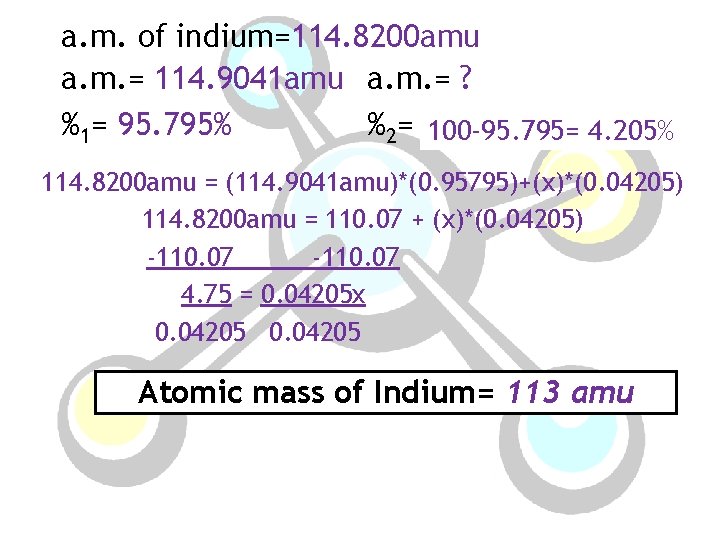
a. m. of indium=114. 8200 amu a. m. = 114. 9041 amu a. m. = ? %1= 95. 795% %2= ? 100 -95. 795= 4. 205% 114. 8200 amu = (114. 9041 amu)*(0. 95795)+(x)*(0. 04205) 114. 8200 amu = 110. 07 + (x)*(0. 04205) -110. 07 4. 75 = 0. 04205 x 0. 04205 Atomic mass of Indium= 113 amu