Unit 2 Energy Flow in Technological Systems Chapters






















































- Slides: 92

Unit 2 Energy Flow in Technological Systems Chapters 4 -6 P. 138 -253 And Chapter 10 P. 360 -398 SNAP P. 87 -155

In this unit we will explore: • Basic concepts of thermodynamics and mechanics • Types of energy • Energy conversions • Analysis of motion • Specific Heat Capacity • Heat of Fusion and Heat of Vaporization • Social and environmental issues

• You will need to bring with you every day: – A pen and pencil – A calculator – A ruler – A protractor Note: Answers to Practice Problems from the text are in Apendix C (P. 484)

• Tips for success: – Come prepared. (bring all the appropriate materials) – Take down the examples! – Use your class time wisely. – Do the work. (Physics gets easier with practice. Homework questions often show up on exams)

Intro activity • Baseball bat physics • Volunteer will try and balance a baseball bat on the palm of his/her hand. • Which way is easiest to balance it? Why?

Chapter 4 Thermal Energy and work P. 140 -173 • Read article on “Turkey Power”

4. 1 The Development of Steam Engine • For thousands if years, people have known that when they boil water, the resulting steam exerts pressure and moves objects. • Hero’s steam engine (Pg 142) – Why did the steam cause the ball to spin? • Do Find Out Activity page 143 -Demo

Steam Engines • Steam engines are machines that generate steam and converts steam pressure into mechanical motion. How does a train steam engine work? How does the train move?

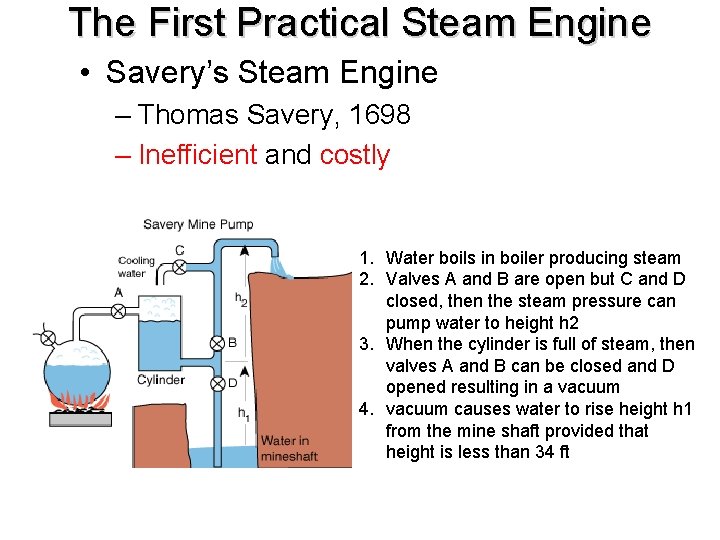
The First Practical Steam Engine • Savery’s Steam Engine – Thomas Savery, 1698 – Inefficient and costly 1. Water boils in boiler producing steam 2. Valves A and B are open but C and D closed, then the steam pressure can pump water to height h 2 3. When the cylinder is full of steam, then valves A and B can be closed and D opened resulting in a vacuum 4. vacuum causes water to rise height h 1 from the mine shaft provided that height is less than 34 ft

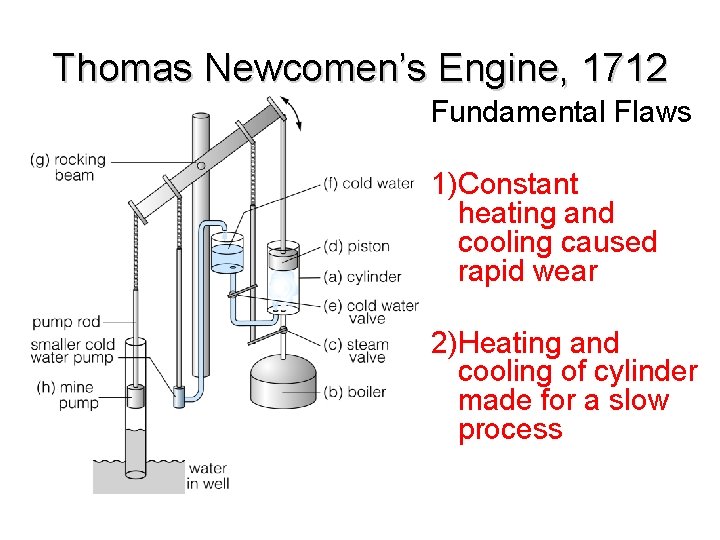
Thomas Newcomen’s Engine, 1712 Fundamental Flaws 1)Constant heating and cooling caused rapid wear 2)Heating and cooling of cylinder made for a slow process

Read pages 144 -145 and answer the following questions. • Watt’s steam engine is sometimes called a “doubleacting” engine. What is acting twice during one cycle? • What is the purpose of the valve in Watt’s steam engine? • What reduces wear and tear on the piston in Watt’s steam engine? • How did Watt adapt his steam engine to perform tasks other than pumping water out of mines?

Read pages 144 -145 and answer the following questions. • Watt’s steam engine is sometimes called a “double-acting” engine. What is acting twice during one cycle? – The pressure pushes the piston both ways. Since steam acts on the piston twice in one complete cycle, the engine is called a “double-acting” engine. • What is the purpose of the valve in Watt’s steam engine? – It moves one way to direct the steam to one side of the piston and then moves the other way to direct the steam to the opposite side of the piston. • What reduces wear and tear on the piston in Watt’s steam engine? – The cylinder in Watt’s steam engine is always hot, the cylinder and piston are not subjected to frequent heating and cooling. • How did Watt adapt his steam engine to perform tasks other than pumping water out of mines? – Watt invented systems of levers and a crankshaft that allowed the steam engine to turn a large wheel. A belt connected the wheel of the engine to other wheels, which could run many types of machines.

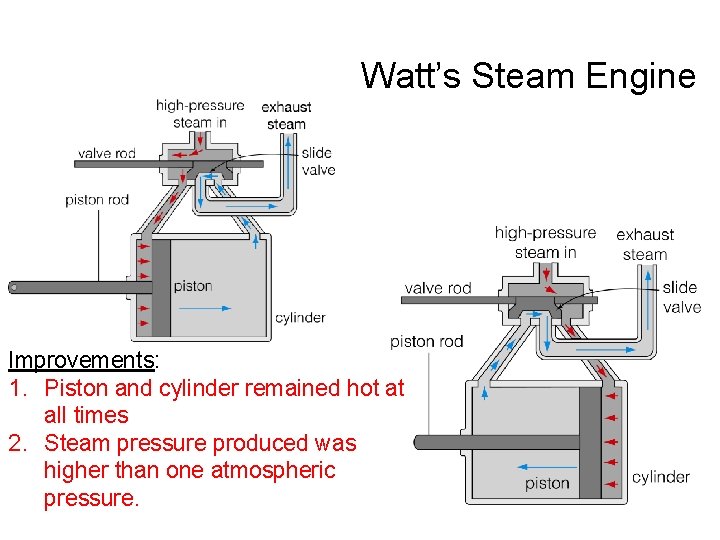
Watt’s Steam Engine Improvements: 1. Piston and cylinder remained hot at all times 2. Steam pressure produced was higher than one atmospheric pressure.

Steam Engines and the Industrial Revolution • Watt’s steam engine was adapted to drive many types of machinery and was responsible for the rapid development of the Industrial Revolution.

FYI • Many steam engines of the day, including Savery, Newcomen, and Watt’s used burning coal or wood as a source of energy. • Today steam engines are not used for locomotives, however, steam turbines still power ocean liners and cruise ships.

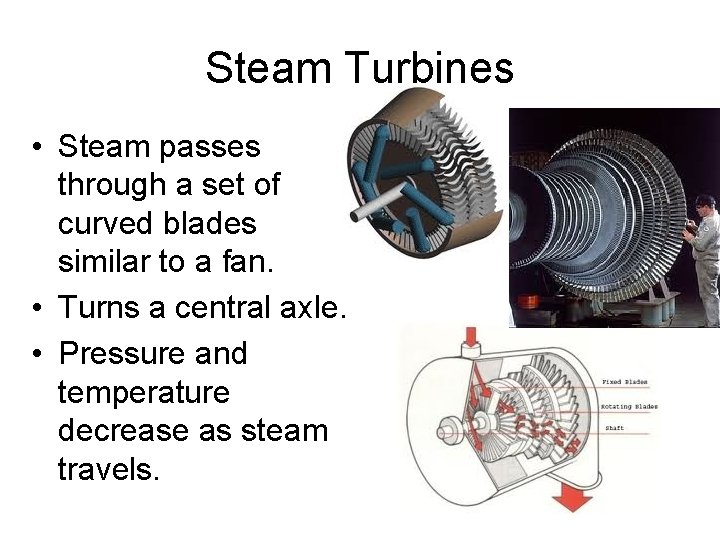
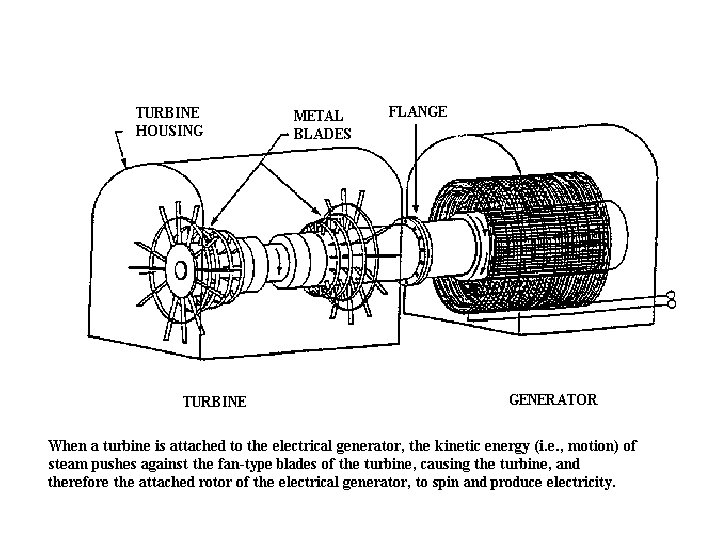
Steam Turbines • Steam passes through a set of curved blades similar to a fan. • Turns a central axle. • Pressure and temperature decrease as steam travels.

• Do Check Your Understanding P. 149. # 1 -6

4. 2 Scientific theories of Heat P. 150 - 163

Early Theories of Heat • Each early theory was an important step in the development of scientific knowledge. Theories and models were based on the knowledge of the time. • Only when new information became available that a theory was modified or discarded. • What other scientific theories have changed over time?

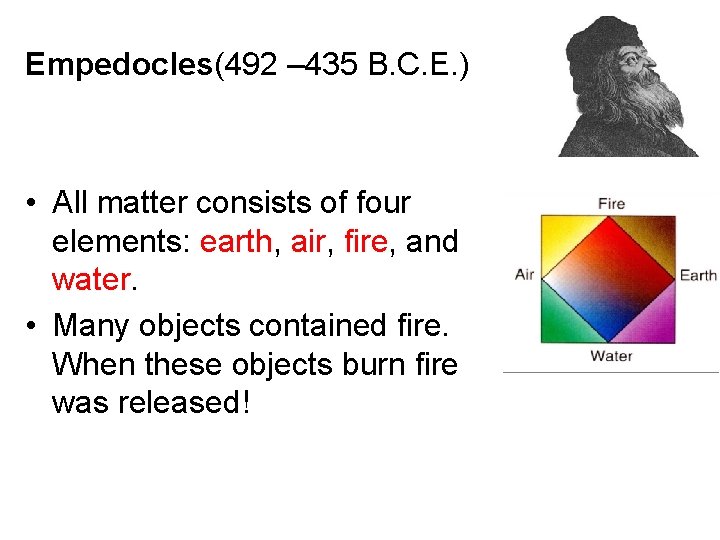
Empedocles(492 – 435 B. C. E. ) • All matter consists of four elements: earth, air, fire, and water. • Many objects contained fire. When these objects burn fire was released!

Phlogiston (Early 1700 s) • Substances that could burn contained an invisible fluid called phlogiston. • The phlogiston flowed out of an object when it burned.

Caloric Theory (Late 1700’s) • Caloric was a mass-less fluid found in all matter. • It could not be created or destroyed, but could flow from one substance to another. • caloric always flows from warmer objects to cooler objects. • Unit – the calorie. • 1 cal. is the quantity of caloric that would increase the temperature of 1 g of water by 1° C.

Modern theories of heat • Benjamin Thompson (1753 -1814) • First to reveal flaw in caloric theory • Was knighted and became known as Count Rumford.

Modern Theories of Heat Rumford Hypothesis (1798) : • Why did Rumford reject the current theory of heat? – Found the flaw by when a hole was bored into metal to make a cannon, the tools, metal and metal shavings became hot. Since none of the materials had been hot when the boring process began, what is the source of the caloric, or heat? Caloric is supposed to flow from warm to cool?

Rumford Hypothesis (1798) • • There is no substance such as caloric. some mechanical energy is converted into heat. “ heat is equivalent to energy. ”

Julius Robert Mayer (1840) • • Found evidence supporting Rumford through blood letting. one of the first scientists to recognize that the body uses oxygen to break down food and use it for energy. He reasoned that the same processes must also be providing heat. “Heat is related to energy”

James prescott joules (1818 - 1889) Mayer wrote a paper on his discovery but it was overlooked due to poor quality of presentation. James Joules, a physicist, also presented the same theory. Ended up receiving the credit for discovering the mechanical equivalent of heat. SI unit for energy named after him.

Energy and Work (pages 153 -155) • Energy= the ability to do work • Work= the transfer of mechanical energy from one object to another • More than one force act on an object at the same time • Work done by different forces is reported separately.

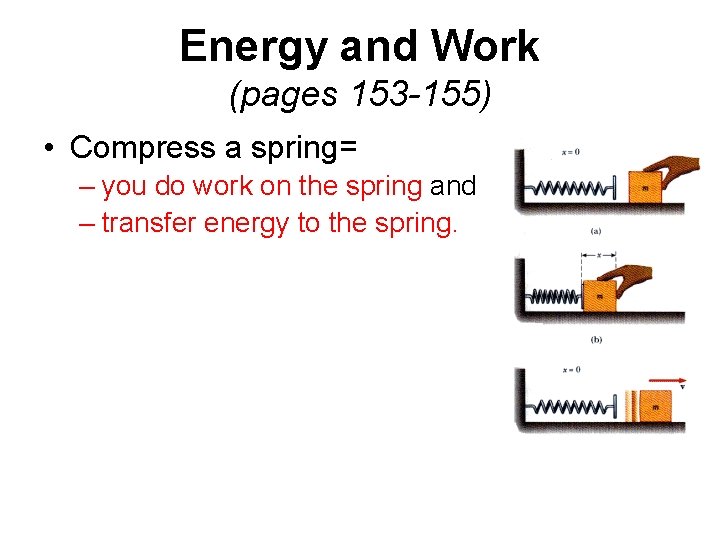
Energy and Work (pages 153 -155) • Compress a spring= – you do work on the spring and – transfer energy to the spring.

W = F • ∆d W is the work in Joules (J) F is the force in Newtons (N) ∆d is the distance in meters (m) Note: 1 J = 1 N • m

Recall: Significant Digits: • The number of significant digits in an answer to a calculation will depend on the number of significant digits in the data. – For multiplication & division, the # of significant digits is determined by the # with the least amount of significant digits in the question. – For addition & subtraction, the number of significant digits is determined by the least precise number. i. e. you use the same amount of decimal places as the number with the least amount of decimal places.

Significant Digits Rules: • Non-zero digits are always significant • With zeroes: – Zeroes placed before other digits are not significant – Zeroes placed between other digits are always significant – Zeroes placed after other digits are significant

Example #1: • You exert 25 N of force on your textbook while lifting it 1. 4 m off the ground and placing it on a shelf. How much work did you do on your textbook?

Example #1: • You exert 25 N of force on your textbook while lifting it 1. 4 m off the ground and placing it on a shelf. How much work did you do on your textbook? F = 25 N ∆d = 1. 4 m W = ?

Example #1: • You exert 25 N of force on your textbook while lifting it 1. 4 m off the ground and placing it on a shelf. How much work did you do on your textbook? F = 25 N ∆d = 1. 4 m W = ? W = F • ∆d = (25 N)(1. 4 m) = 35 J

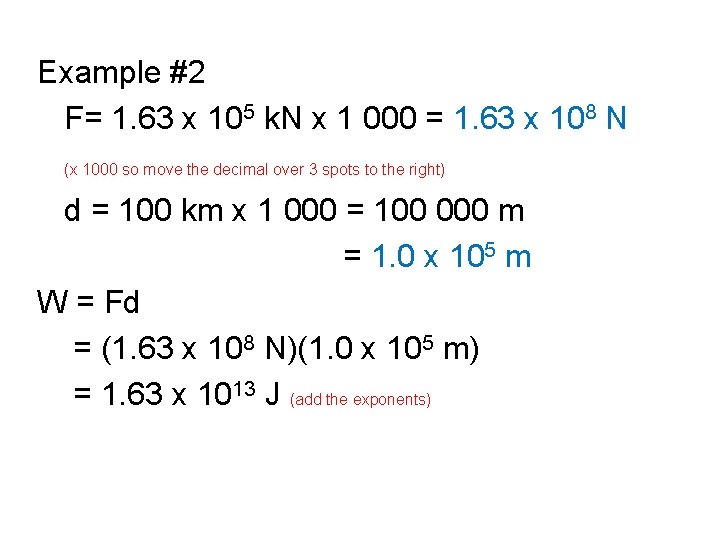
Example #2 Working with Sci. Notation • The edge of space is defined as “the karman line” 100 km above the Earth’s surface. If 1. 63 x 105 k. N of force are required to launch the 2 029 203 kg space shuttle Discovery up to the edge of space, how much work is done?

Example #2 F= 1. 63 x 105 k. N x 1 000 = 1. 63 x 108 N (x 1000 so move the decimal over 3 spots to the right) d = 100 km x 1 000 = 100 000 m = 1. 0 x 105 m W = Fd = (1. 63 x 108 N)(1. 0 x 105 m) = 1. 63 x 1013 J (add the exponents)

• Do Practice Problems 1 to 9 P. 154 • Do BLM 4 -5 energy and Work Practice Problems

You have a long metal tube that is curved. You also have a miniature golf ball that you are knocking into one end and out the other. How does the ball move after it leaves the other end of the tube? Does it move in a straight line or a curve? Perhaps it starts by moving in a curved line, then gradually straightens out? Or something else? A moment in science

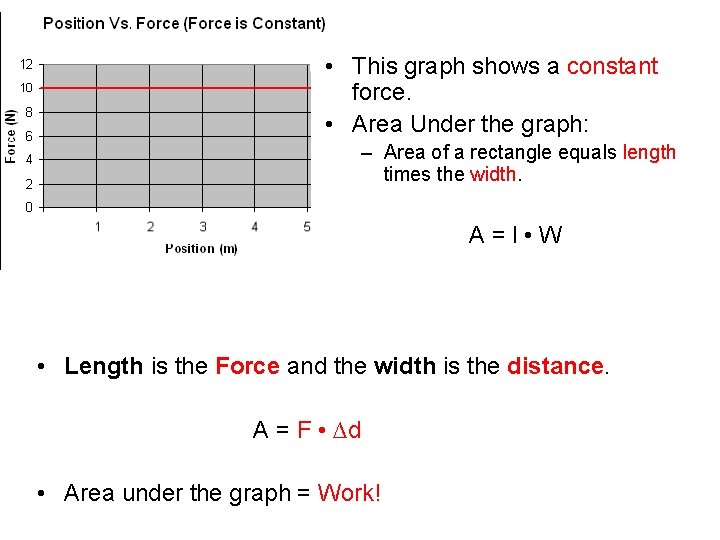
Graphical Methods for Determining Work • Lets look at some position vs. force graphs. . .

• This graph shows a constant force. • Area Under the graph: – Area of a rectangle equals length times the width. A = l • W • Length is the Force and the width is the distance. A = F • ∆d • Area under the graph = Work!

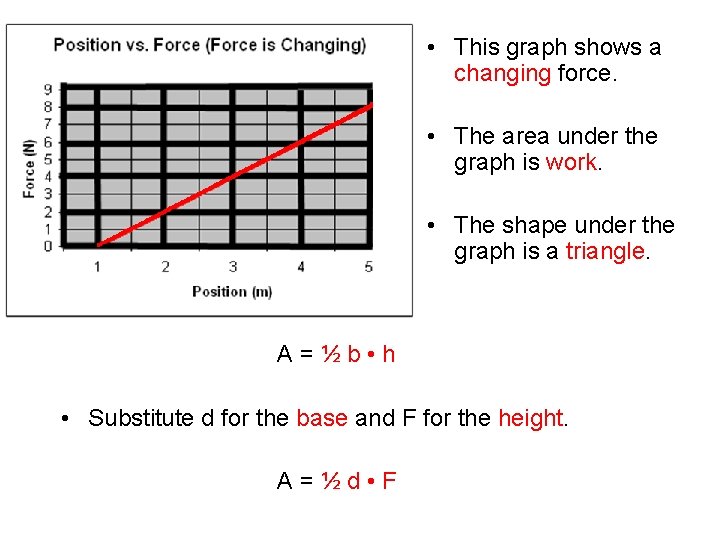
• This graph shows a changing force. • The area under the graph is work. • The shape under the graph is a triangle. A = ½ b • h • Substitute d for the base and F for the height. A = ½ d • F

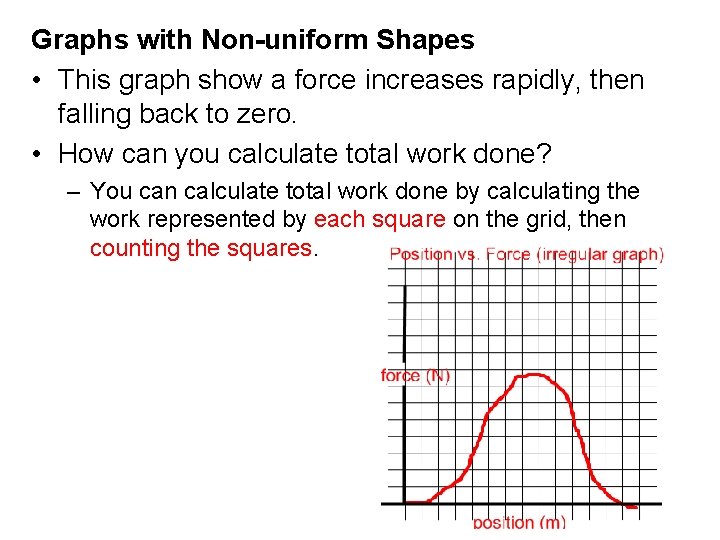
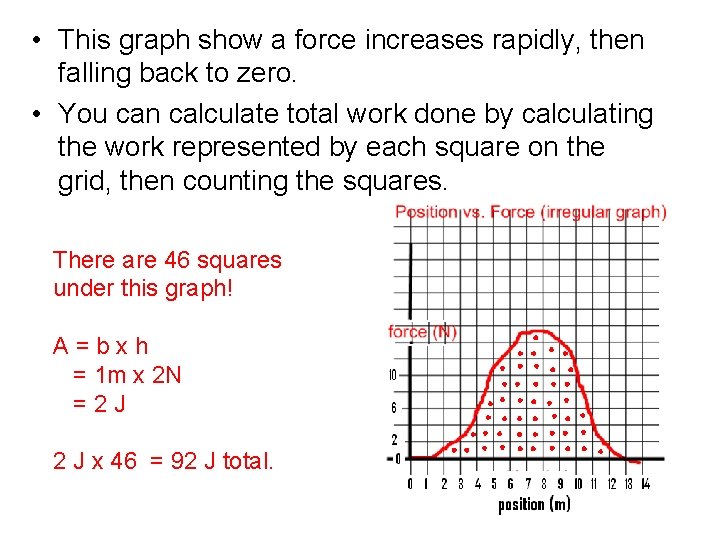
Graphs with Non-uniform Shapes • This graph show a force increases rapidly, then falling back to zero. • How can you calculate total work done? – You can calculate total work done by calculating the work represented by each square on the grid, then counting the squares.

• This graph show a force increases rapidly, then falling back to zero. • You can calculate total work done by calculating the work represented by each square on the grid, then counting the squares. There are 46 squares under this graph! A = b x h = 1 m x 2 N = 2 J x 46 = 92 J total.

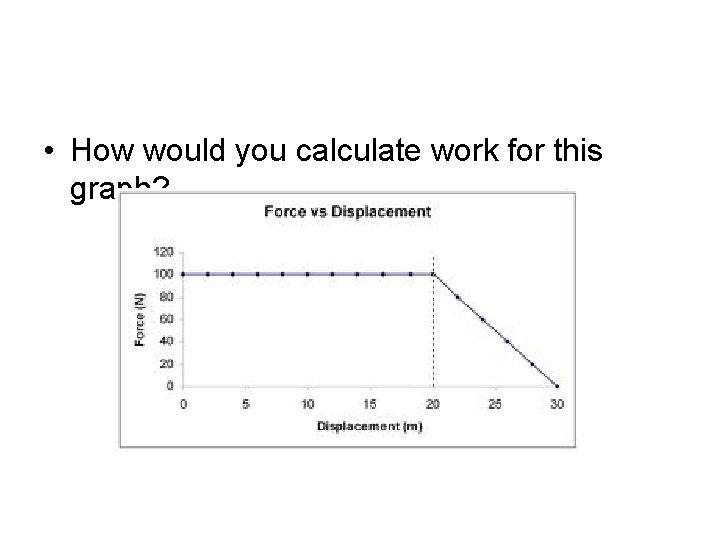
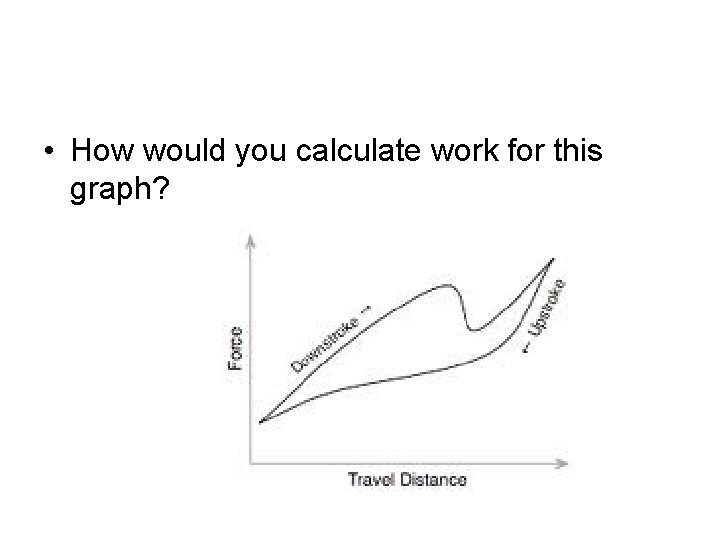
• How would you calculate work for this graph?

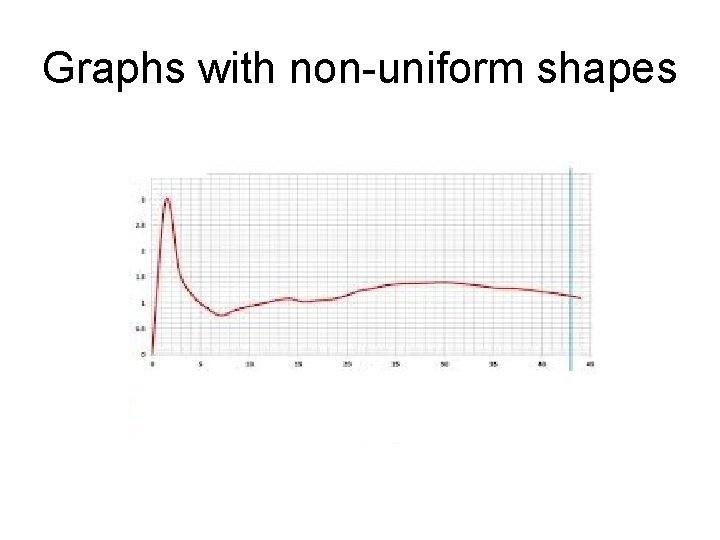
Graphs with non-uniform shapes

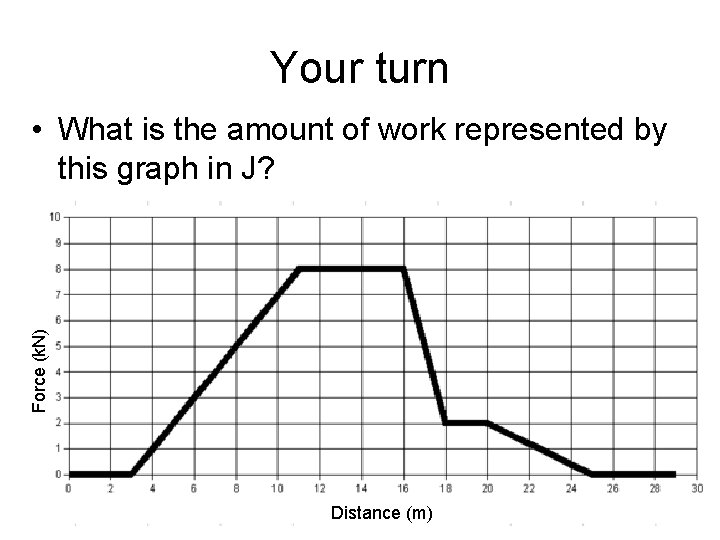
Your turn Force (k. N) • What is the amount of work represented by this graph in J? Distance (m)

• How would you calculate work for this graph?

• Do Practice Problems 10 and 11 page 157 – 158 • Do BLM 4 -6 Graphical Methods for Determining work

A moment in Science Energy is neither created or destroyed: but it can be transformed from one form to another or one object to another.

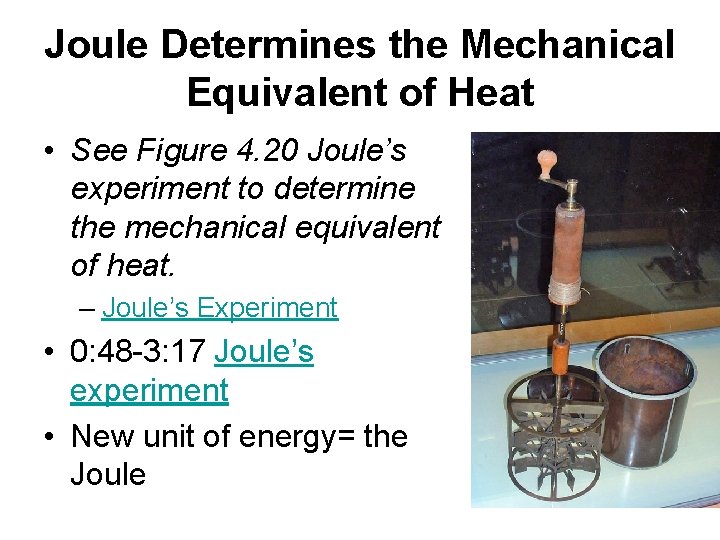
Joule Determines the Mechanical Equivalent of Heat • See Figure 4. 20 Joule’s experiment to determine the mechanical equivalent of heat. – Joule’s Experiment • 0: 48 -3: 17 Joule’s experiment • New unit of energy= the Joule

• The work of Mayer and Joule led to the law of conservation of energy. • “energy cannot be created or destroyed, but can be converted from one form to another. ”

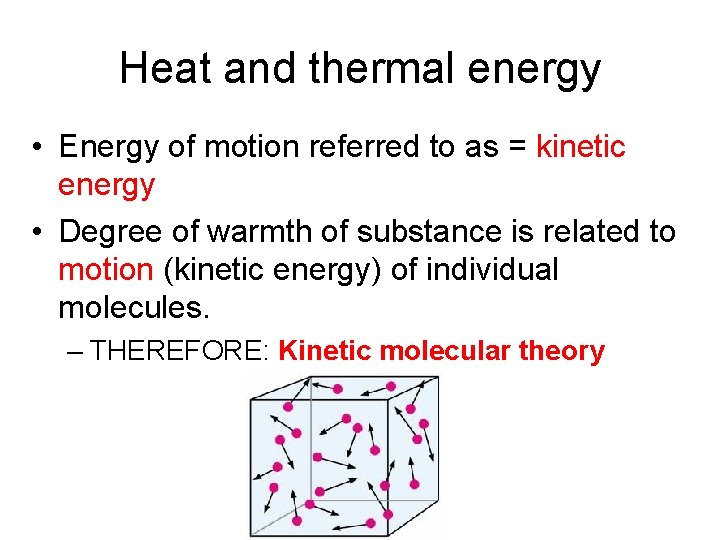
The Kinetic-Molecular Theory • Atoms are in random motion and collide with each other, transferring energy Work. • Work and Energy are related! • Average speed of molecules high = substance is hot

Heat and thermal energy • Energy of motion referred to as = kinetic energy • Degree of warmth of substance is related to motion (kinetic energy) of individual molecules. – THEREFORE: Kinetic molecular theory

Heat and thermal energy • Heat: – The transfer of thermal energy from one object to another • Heat and work are mechanisms by which energy can be transferred from one object to another.

Heat and Thermal Energy Some Definitions: • Kinetic energy : Energy of motion • Kinetic-molecular energy : energy of molecules in constant random motion, aka thermal energy • Heat : Transfer of thermal energy from one object to another • Temperature: Average kinetic energy of the atoms or molecules in a substance. • Work : Transfer of mechanical energy from one object to another

Specific heat capacity (10. 2) • Joules experiment found that in order to to increase the temperature of water by 1°C you would need to do 4. 16 J of work. – Actual 4. 186 J – How would you do this work?

Specific Heat Capacity • amount of energy (J) to increase the temperature of 1 g of the substance by 1˚C. • Symbol c • Unit J/g˚C

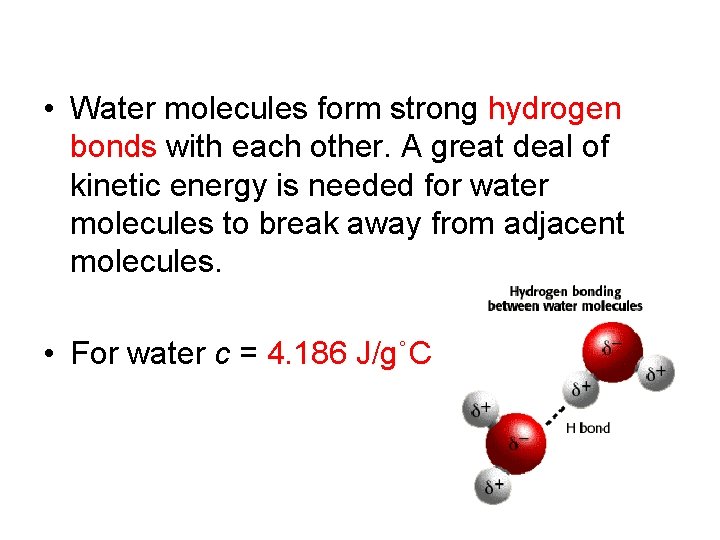
• Water molecules form strong hydrogen bonds with each other. A great deal of kinetic energy is needed for water molecules to break away from adjacent molecules. • For water c = 4. 186 J/g˚C

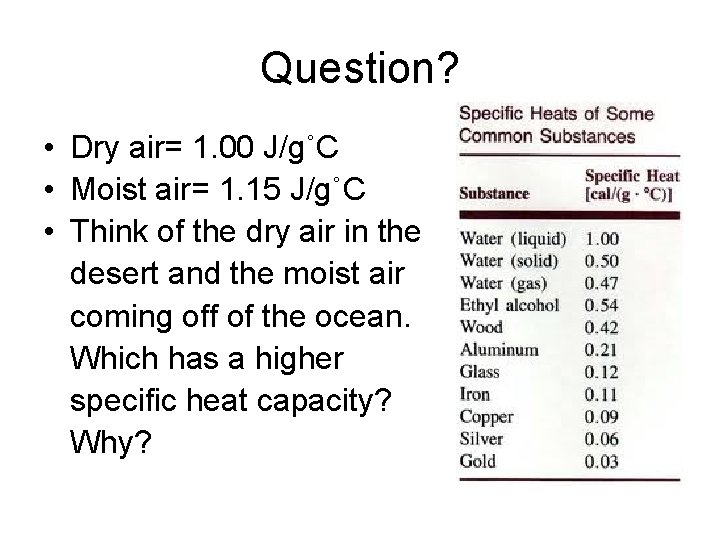
Question? • Dry air= 1. 00 J/g˚C • Moist air= 1. 15 J/g˚C • Think of the dry air in the desert and the moist air coming off of the ocean. Which has a higher specific heat capacity? Why?

• Cold moist air feels colder than dry cold air (and hot moist air feels hotter than hot dry air) because moist air has a higher specific heat than does dry air • It takes more energy to heat moist air. • Why then do we encourage moist air (humid) in most climates?

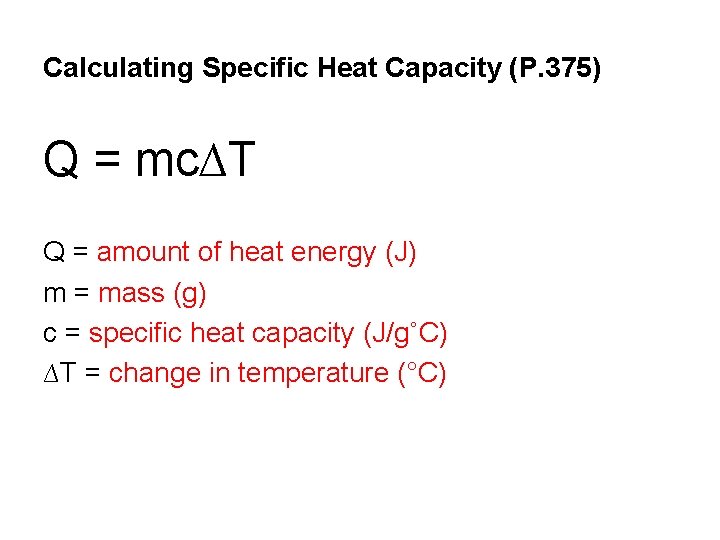
Calculating Specific Heat Capacity (P. 375) Q = mc∆T Q = amount of heat energy (J) m = mass (g) c = specific heat capacity (J/g˚C) ∆T = change in temperature (°C)

• Note: ∆T is the change in temperature, so if two temperatures are given in the problem, you must subtract them using the formula T 1 - T 2 = ∆T • A list of Specific heat capacities is given on P. 375 in table 10. 1 and on the back of your formula sheet.

Example 1: • A house contains 170 Kg of dry air. The furnace is broken and the temperature in the house is 2. 0°C. How much energy is needed to heat the air to 20°C ?

Example 1: • A house contains 170 Kg of dry air. The furnace is broken and the temperature in the house is 2. 0°C. How much energy is needed to heat the air to 20°C ? Q = ? (J) m = 170 Kg = 170 000 g c = 1. 00 J/g˚C (from chart) ∆T = 20°C – 2. 0°C = 18°C

Q = ? (J) m = 170 Kg = 170 000 g c = 1. 00 J/g˚C (from chart) ∆T = 20°C – 2. 0°C = 18°C Q = mc∆T = (170 000 g)(1. 00 J/g˚C)(18°C)

Q = ? (J) m = 170 Kg = 170 000 g c = 1. 00 J/g˚C (from chart) ∆T = 20°C – 2. 0°C = 18°C Q = mc∆T = (170 000 g)(1. 00 J/g˚C)(18°C) = 3 060 000 J = 3. 1 x 106 J or 3. 1 MJ

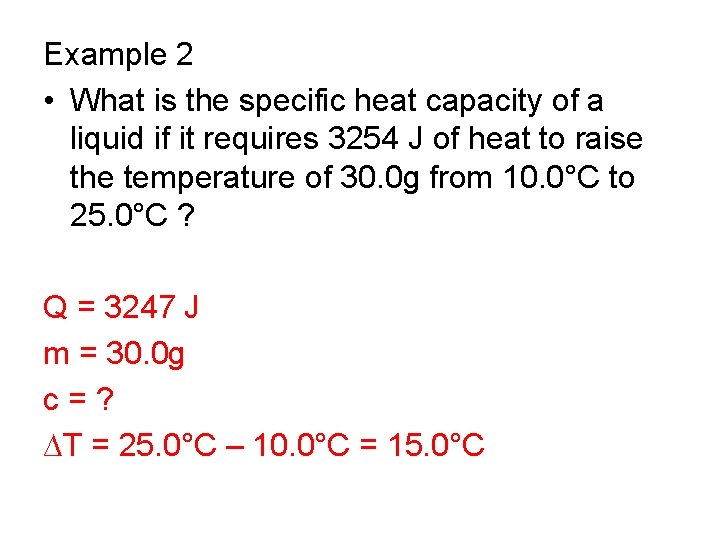
Example 2 • What is the specific heat capacity of a liquid if it requires 3254 J of heat to raise the temperature of 30. 0 g from 10. 0°C to 25. 0°C ?

Example 2 • What is the specific heat capacity of a liquid if it requires 3254 J of heat to raise the temperature of 30. 0 g from 10. 0°C to 25. 0°C ? Q = 3247 J m = 30. 0 g c = ? ∆T = 25. 0°C – 10. 0°C = 15. 0°C

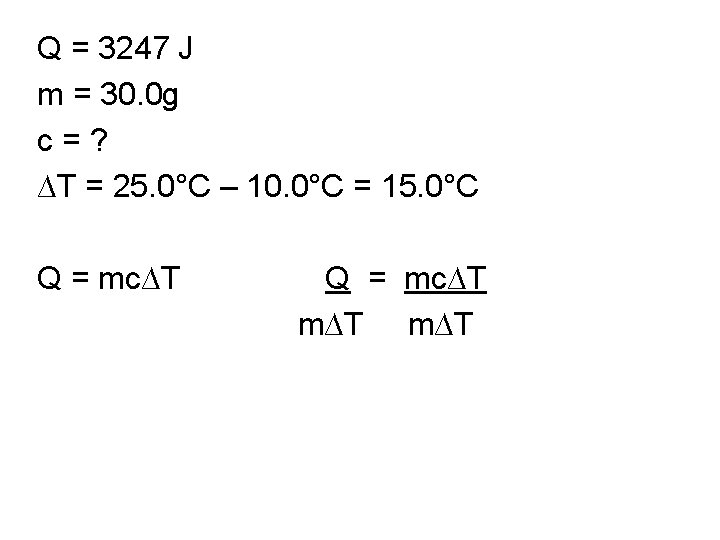
Q = 3247 J m = 30. 0 g c = ? ∆T = 25. 0°C – 10. 0°C = 15. 0°C Q = mc∆T

Q = 3247 J m = 30. 0 g c = ? ∆T = 25. 0°C – 10. 0°C = 15. 0°C Q = mc∆T m∆T

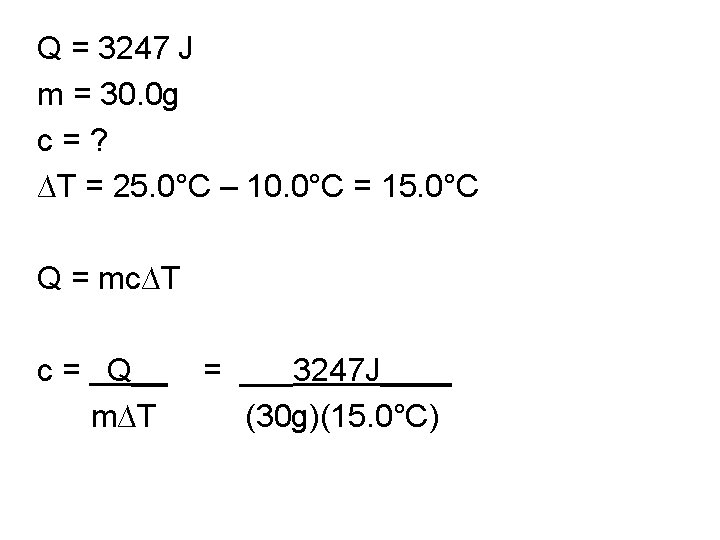
Q = 3247 J m = 30. 0 g c = ? ∆T = 25. 0°C – 10. 0°C = 15. 0°C Q = mc∆T c = Q__ m∆T

Q = 3247 J m = 30. 0 g c = ? ∆T = 25. 0°C – 10. 0°C = 15. 0°C Q = mc∆T c = Q__ = ___3247 J____ m∆T (30 g)(15. 0°C)

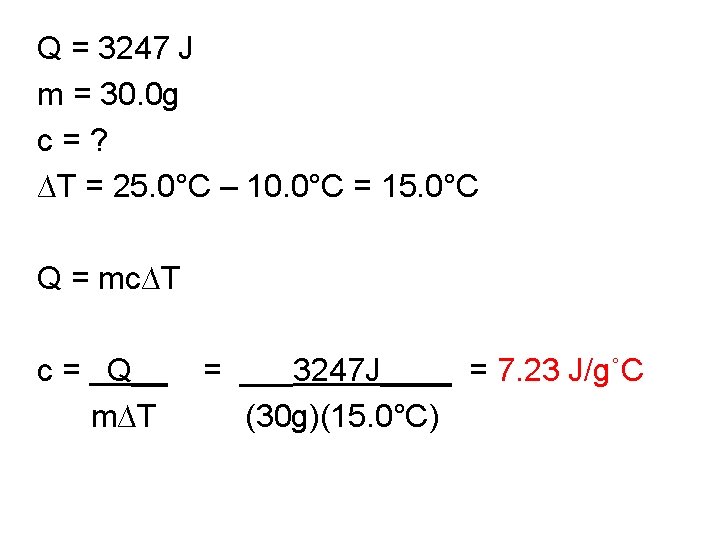
Q = 3247 J m = 30. 0 g c = ? ∆T = 25. 0°C – 10. 0°C = 15. 0°C Q = mc∆T c = Q__ = ___3247 J____ = 7. 23 J/g˚C m∆T (30 g)(15. 0°C)

• Now do Practice Problems P. 377 #1 -9

• Complete the activity on P. 162 Heat versus Temperature

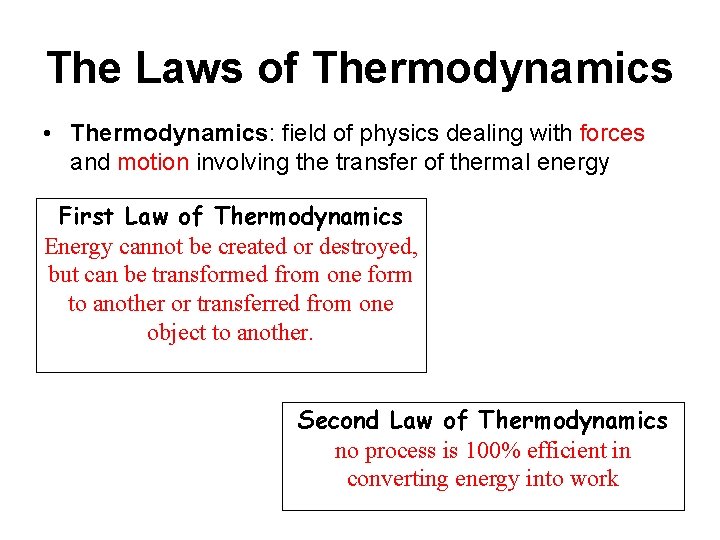
The Laws of Thermodynamics • Thermodynamics: field of physics dealing with forces and motion involving the transfer of thermal energy First Law of Thermodynamics Energy cannot be created or destroyed, but can be transformed from one form to another or transferred from one object to another. Second Law of Thermodynamics no process is 100% efficient in converting energy into work

• No process can be 100 percent efficient. Some energy will always remain in the form of thermal energy (friction). • Thermal energy always flows from objects at a higher temperature to objects at a lower temperature • Check your Understanding P. 163 # 3, 4, 5, 7, 8, 9, 10

Heat of Fusion and Heat of Vaporization P. 381 • Energy required for melting Q = n. Hfus • Energy required for vaporization Q = n. Hvap Q = heat energy (J) n = number of moles (mol) Hfus = heat of fusion (J/mol) Hvap = heat of vaporization (J/mol) *values are give in tbl. 10. 2 on p. 381 and on the back of your formula sheet.

• If 20 J of energy is needed to melt a substance, 20 J of energy are released when it freezes. • Remember the first law of thermodynamics. • Similarly, the amount of energy required to vaporize a substance is equal to the amount of energy released when it condenses back into a liquid.


Heat of Vaporization • Example – A beaker containing exactly 360. 4 g of water in a the liquid state at 100°C. How much energy is required to convert the liquid to water vapor at 100°C?

Heat of Vaporization • Example – A beaker containing exactly 360. 4 g of water in a the liquid state at 100°C. How much energy is required to convert the liquid to water vapor at 100°C? n = m/M n = 360. 4 g 18. 02 g/mol = 20. 00 mol

Heat of Vaporization • Example – A beaker containing exactly 360. 4 g of water in a the liquid state at 100°C. How much energy is required to convert the liquid to water vapor at 100°C? Hvap = 40. 65 k. J/mol n = m/M Q = n. Hvap n = 360. 4 g = (20. 00 mol)(40. 65 18. 02 g/mol k. J/mol) = 20. 00 mol = 813. 0 k. J

• • Study model problem 2 on p. 382 Do practice problems P. 383 # 10 -18 and P. 387 #3.

4. 3 Sources of Energy for Modern Technologies P. 164

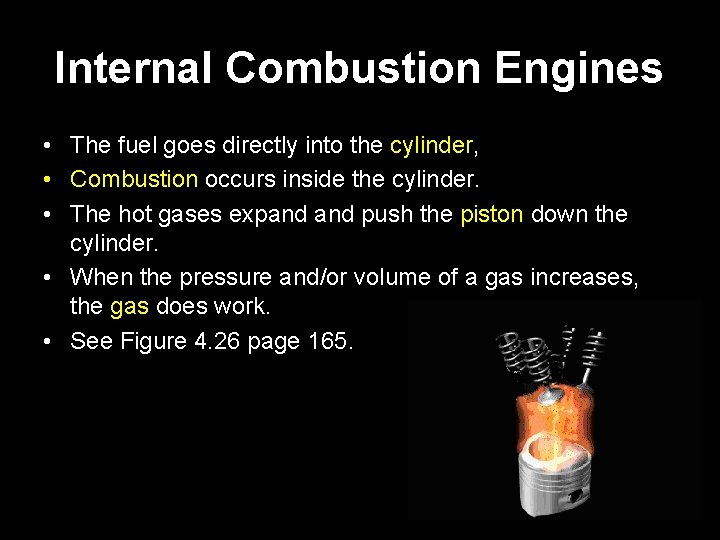
Internal Combustion Engines • The fuel goes directly into the cylinder, • Combustion occurs inside the cylinder. • The hot gases expand push the piston down the cylinder. • When the pressure and/or volume of a gas increases, the gas does work. • See Figure 4. 26 page 165.


Production of Electrical Energy Major sources of energy in Canadian Provinces • Québec and British Colombia: Hydro-electric • Ontario: Half is generated by nuclear reactor • Alberta and Saskatchewan: Coal burning plants


• Do Investigation 4 -B: Producing Electrical Energy page 169

• Do Check your Understanding page 170 questions 1, 2, 3 • Do Chapter at a glance page 171 questions g, j, k, l, m • Do chapter 4 Review page 172 -173 # 1 to 16 and 21 to 26