Ultraviolet Photodissociation Dynamics of the 3 Cyclohexenyl Radical


- Slides: 15

Ultraviolet Photodissociation Dynamics of the 3 -Cyclohexenyl Radical Michael Lucas, Yanlin Liu, Jasmine Minor, Raquel Bryant, Jingsong Zhang Department of Chemistry University of California, Riverside 69 th International Symposium on Molecular Spectroscopy 6/17/2014

Cyclohexyenl Radical Cycloalkanes are important component of conventional fuels Cyclohexane model cycloalkane �Major producer of benzene Previous Research: cyclohexyl, phenyl What effect does the double bond have on the photochemistry?

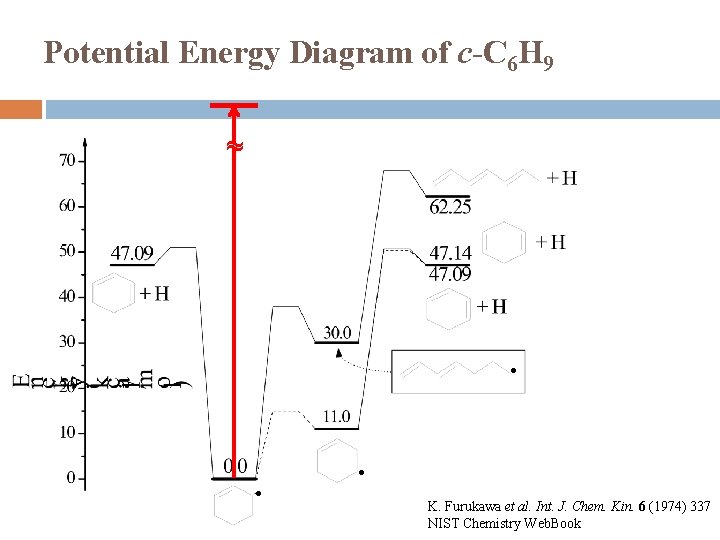
Potential Energy Diagram of c-C 6 H 9 ~ ● ● ● K. Furukawa et al. Int. J. Chem. Kin. 6 (1974) 337 NIST Chemistry Web. Book

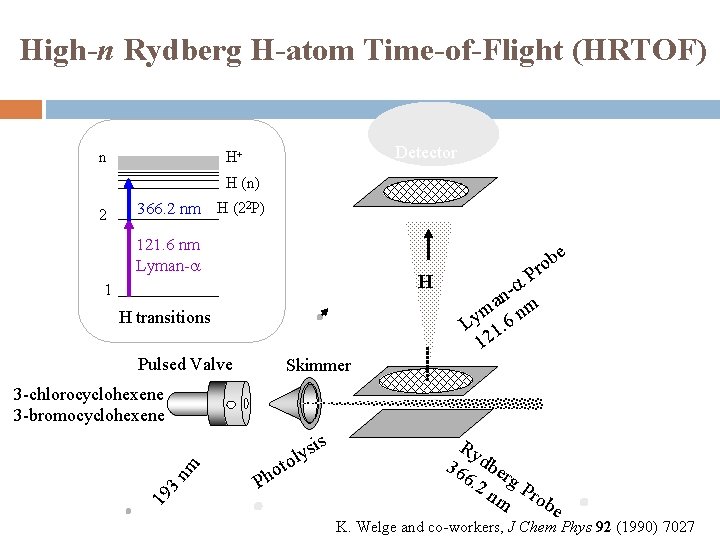
High-n Rydberg H-atom Time-of-Flight (HRTOF) n Detector H+ H (n) 2 366. 2 nm H (22 P) 121. 6 nm Lyman-a e H 1 H transitions Pulsed Valve ob r P -a n ma nm y L 1. 6 12 Skimmer 19 3 nm 3 -chlorocyclohexene 3 -bromocyclohexene o Ph to s i s ly Ry 36 dber 6. 2 g nm Prob e K. Welge and co-workers, J Chem Phys 92 (1990) 7027

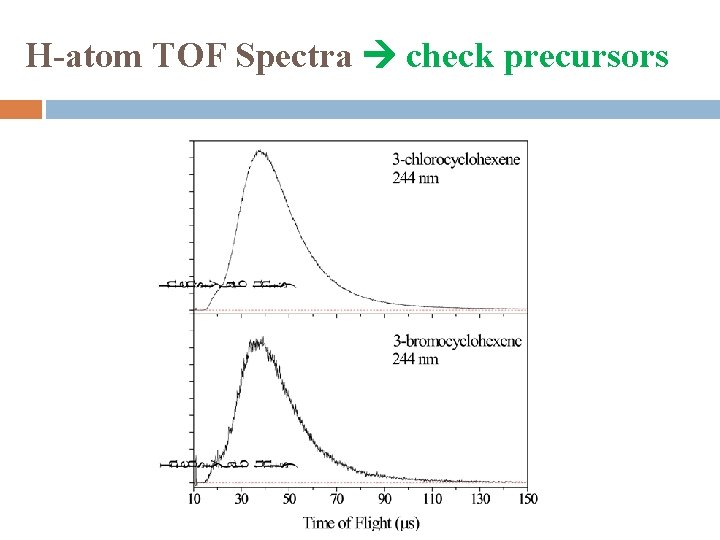
H-atom TOF Spectra check precursors

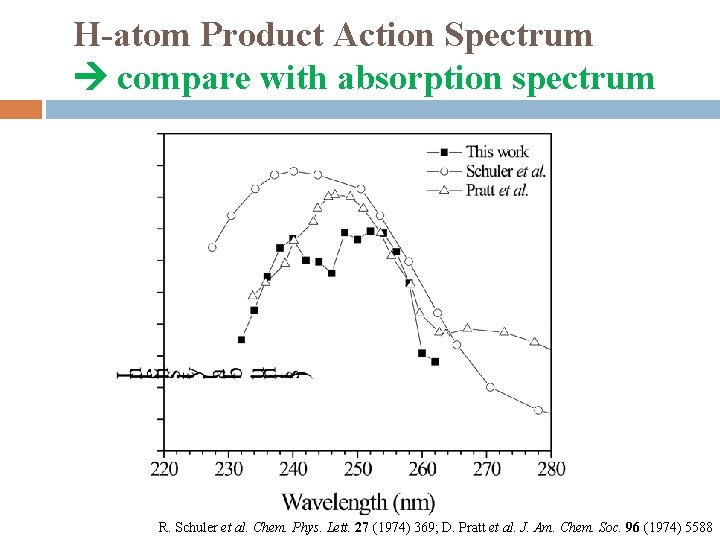
H-atom Product Action Spectrum compare with absorption spectrum R. Schuler et al. Chem. Phys. Lett. 27 (1974) 369; D. Pratt et al. J. Am. Chem. Soc. 96 (1974) 5588

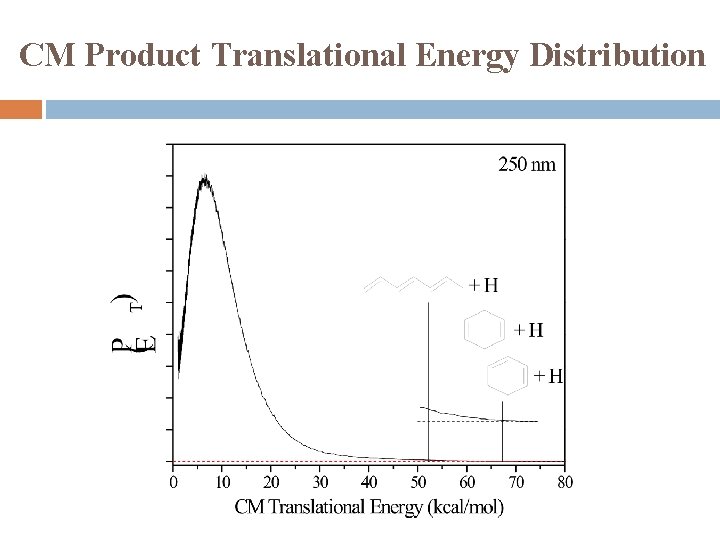
CM Product Translational Energy Distribution

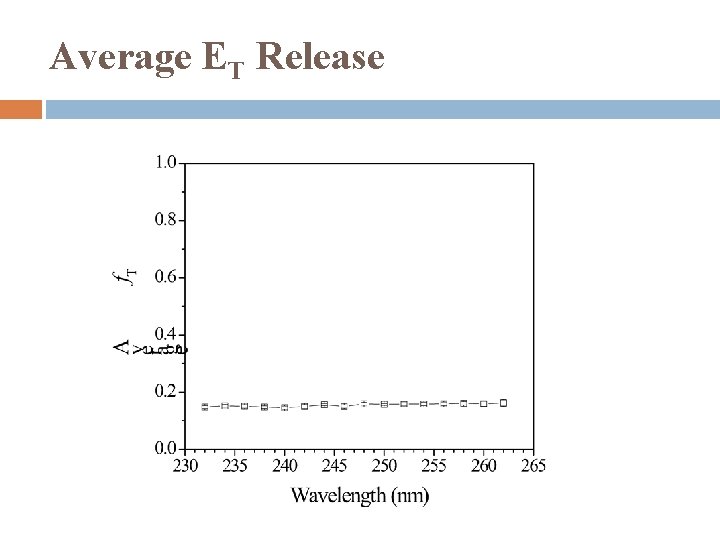
Average ET Release

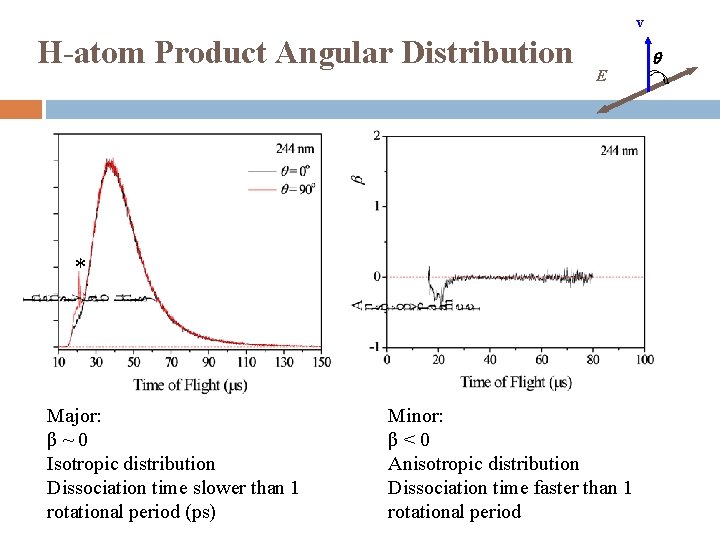
v H-atom Product Angular Distribution q E * Major: β~0 Isotropic distribution Dissociation time slower than 1 rotational period (ps) Minor: β<0 Anisotropic distribution Dissociation time faster than 1 rotational period

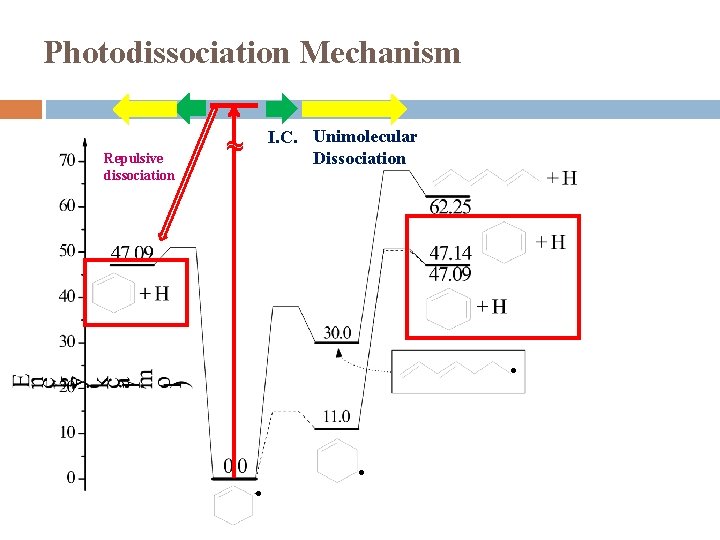
Photodissociation Mechanism Repulsive dissociation I. C. Unimolecular Dissociation ~ ● ● ●

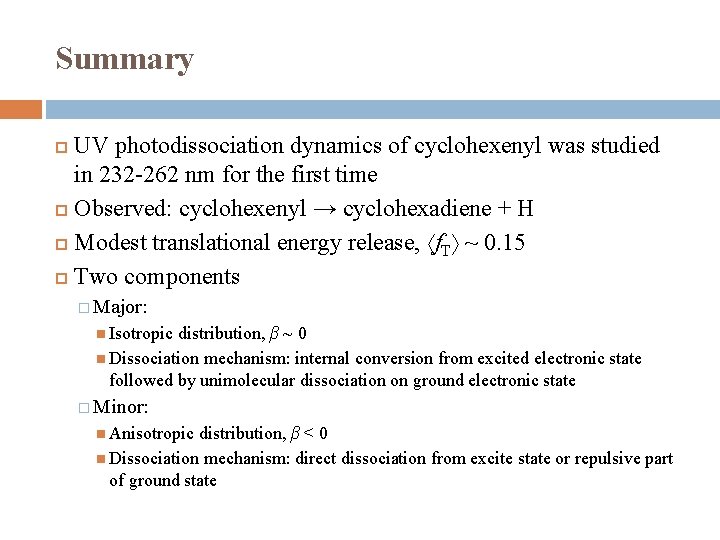
Summary UV photodissociation dynamics of cyclohexenyl was studied in 232 -262 nm for the first time Observed: cyclohexenyl → cyclohexadiene + H Modest translational energy release, f. T ~ 0. 15 Two components � Major: Isotropic distribution, β ~ 0 Dissociation mechanism: internal conversion from excited electronic state followed by unimolecular dissociation on ground electronic state � Minor: Anisotropic distribution, β < 0 Dissociation mechanism: direct dissociation from excite state or repulsive part of ground state

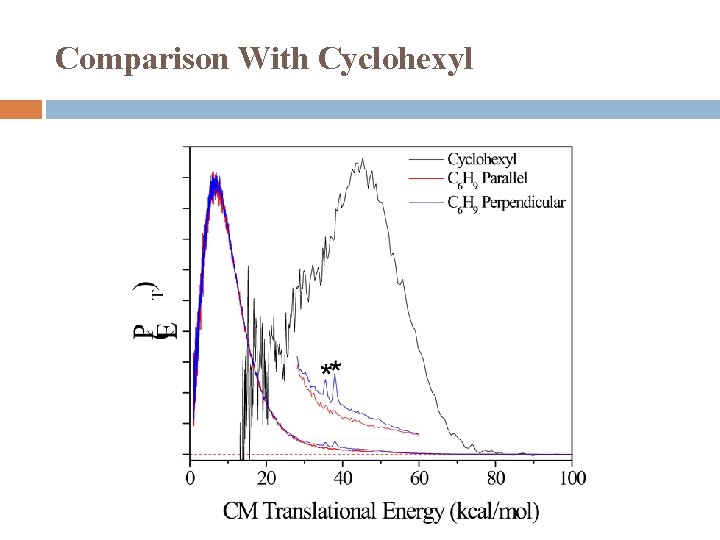
Comparison With Cyclohexyl

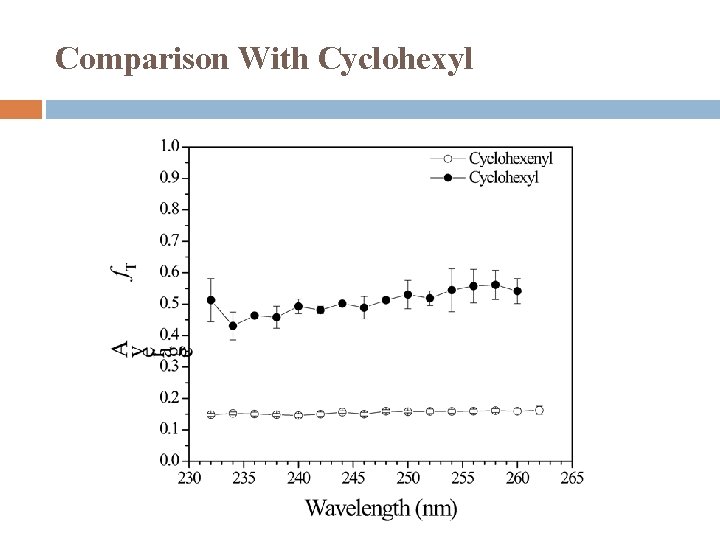
Comparison With Cyclohexyl

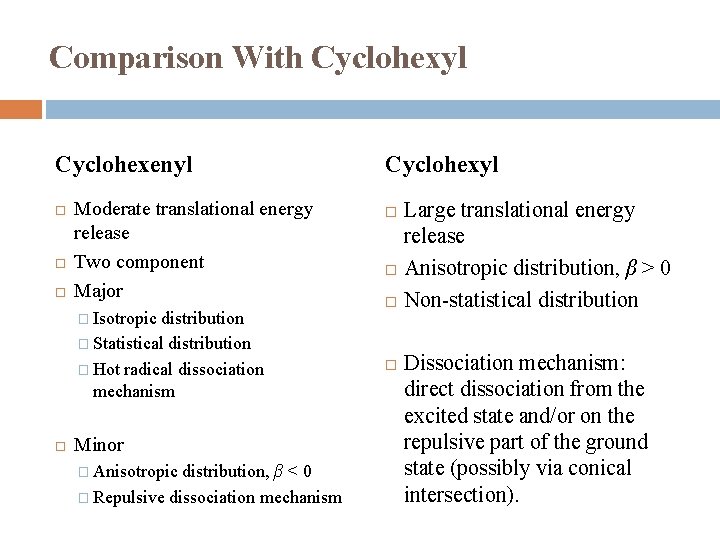
Comparison With Cyclohexyl Cyclohexenyl Moderate translational energy release Two component Major � Isotropic distribution � Statistical distribution � Hot radical dissociation mechanism Minor � Anisotropic distribution, β < 0 � Repulsive dissociation mechanism Cyclohexyl Large translational energy release Anisotropic distribution, β > 0 Non-statistical distribution Dissociation mechanism: direct dissociation from the excited state and/or on the repulsive part of the ground state (possibly via conical intersection).

Acknowledgements Prof. Jingsong Zhang Yanlin Liu Jasmine Minor Raquel Bryant Zhang Group
 Photodissociation
Photodissociation Entire radical
Entire radical Entire radical to mixed radical
Entire radical to mixed radical Unit 6 radical functions homework 4 rational exponents
Unit 6 radical functions homework 4 rational exponents Ultraviolet catastrophe
Ultraviolet catastrophe Uv photography in forensic science
Uv photography in forensic science H2co shape
H2co shape Pengertian penginderaan jauh menurut sabins
Pengertian penginderaan jauh menurut sabins Mnemonic for electromagnetic spectrum
Mnemonic for electromagnetic spectrum Panjang gelombang inframerah
Panjang gelombang inframerah Euv lithography ppt
Euv lithography ppt Ultraviolet amplitude
Ultraviolet amplitude Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Hệ hô hấp
Hệ hô hấp