Photodissociation dynamics of 1 propenyl radical Michael Lucas

- Slides: 17

Photodissociation dynamics of 1 -propenyl radical Michael Lucas, Yu Song, Jingsong Zhang*, Department of Chemistry University of California, Riverside, CA 92521 Christopher Brazier Department of Chemistry and Biochemistry California State University, Long Beach, CA 90840

Photodissociation of Free Radicals Free radicals Open shell Highly reactive Important to many areas of chemistry Combustion, plasma, atmospheric, interstellar Dissociation depends on potential energy surfaces Multiple low-lying electronic states and nonadiabatic processes Provide benchmarks for theory

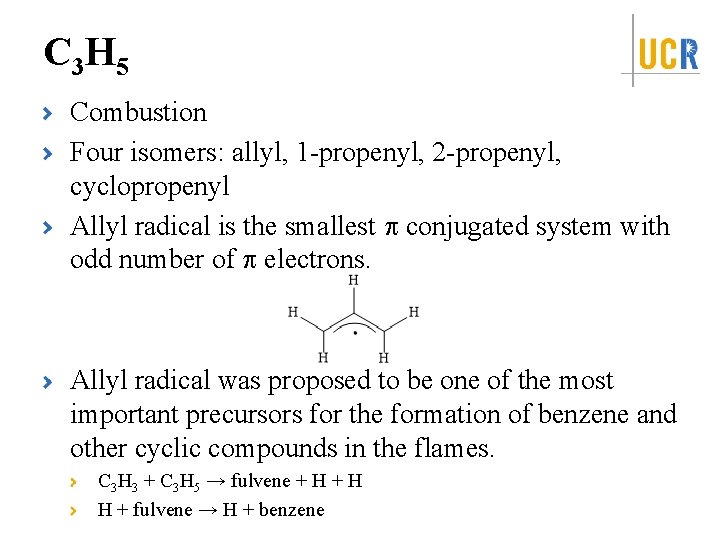
C 3 H 5 Combustion Four isomers: allyl, 1 -propenyl, 2 -propenyl, cyclopropenyl Allyl radical is the smallest conjugated system with odd number of electrons. Allyl radical was proposed to be one of the most important precursors for the formation of benzene and other cyclic compounds in the flames. C 3 H 3 + C 3 H 5 → fulvene + H H + fulvene → H + benzene

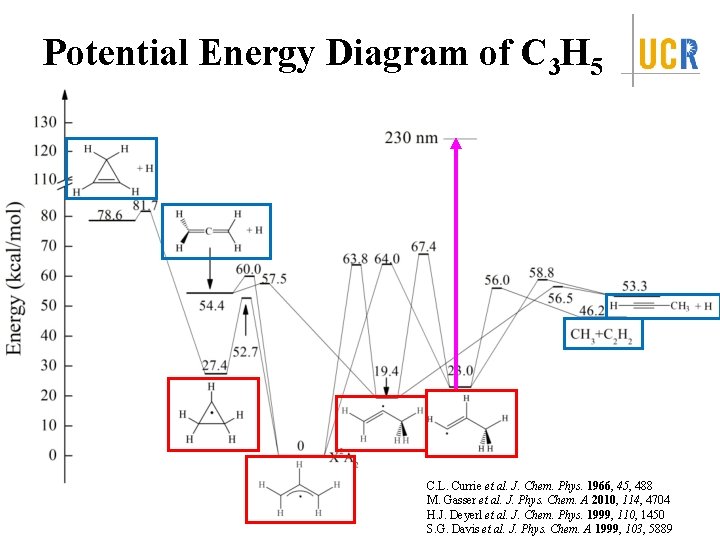
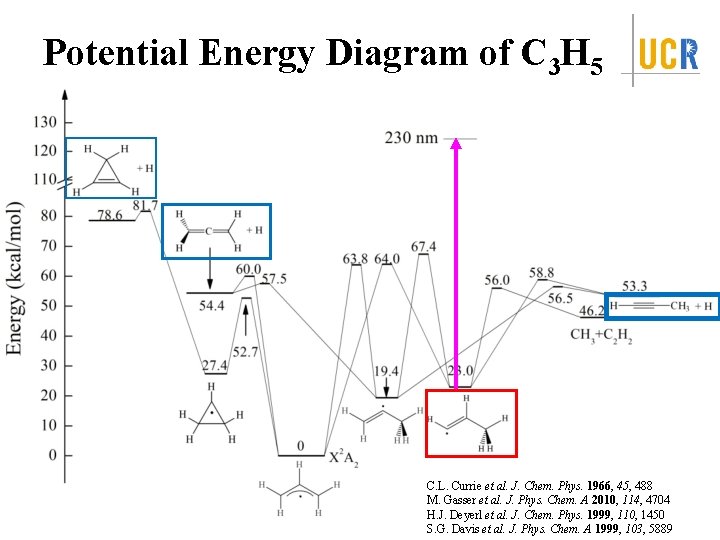
Potential Energy Diagram of C 3 H 5 C. L. Currie et al. J. Chem. Phys. 1966, 45, 488 M. Gasser et al. J. Phys. Chem. A 2010, 114, 4704 H. J. Deyerl et al. J. Chem. Phys. 1999, 110, 1450 S. G. Davis et al. J. Phys. Chem. A 1999, 103, 5889

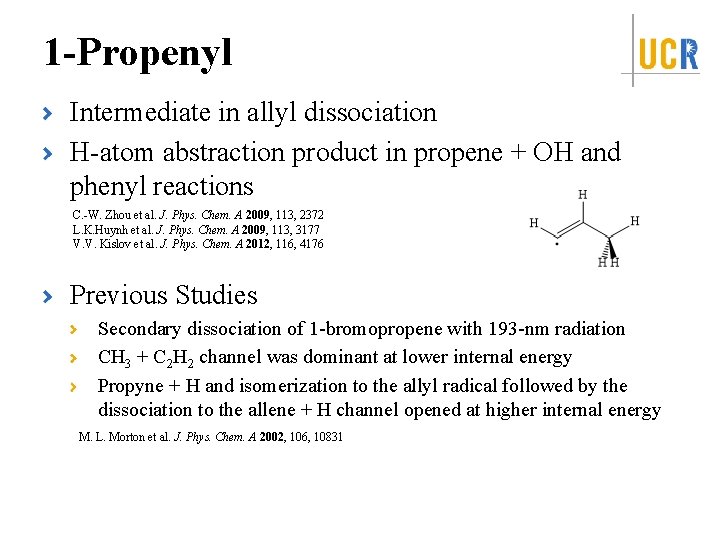
1 -Propenyl Intermediate in allyl dissociation H-atom abstraction product in propene + OH and phenyl reactions C. -W. Zhou et al. J. Phys. Chem. A 2009, 113, 2372 L. K. Huynh et al. J. Phys. Chem. A 2009, 113, 3177 V. V. Kislov et al. J. Phys. Chem. A 2012, 116, 4176 Previous Studies Secondary dissociation of 1 -bromopropene with 193 -nm radiation CH 3 + C 2 H 2 channel was dominant at lower internal energy Propyne + H and isomerization to the allyl radical followed by the dissociation to the allene + H channel opened at higher internal energy M. L. Morton et al. J. Phys. Chem. A 2002, 106, 10831

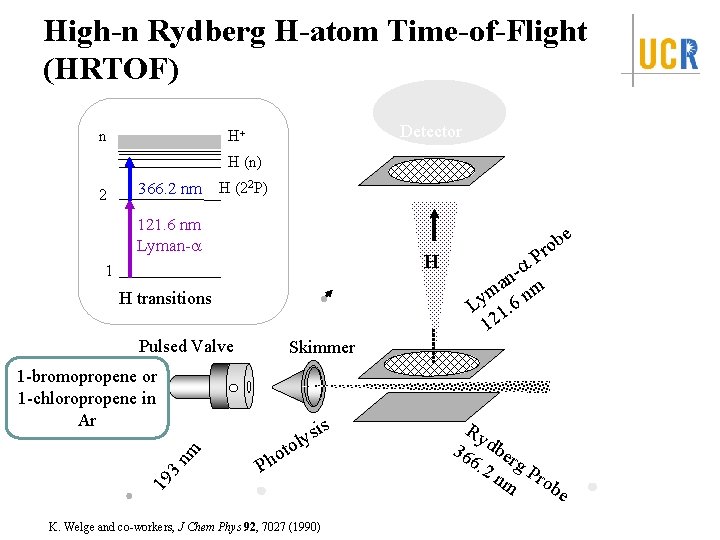
High-n Rydberg H-atom Time-of-Flight (HRTOF) n Detector H+ H (n) 2 366. 2 nm H (22 P) 121. 6 nm Lyman-a e H 1 H transitions Pulsed Valve nm P a na m nm y L 1. 6 12 Skimmer 1 -bromopropene or 1 -chloropropene in Ar s i s ly to o Ph 19 3 b ro K. Welge and co-workers, J Chem Phys 92, 7027 (1990) Ry 36 dber 6. 2 g nm Prob e

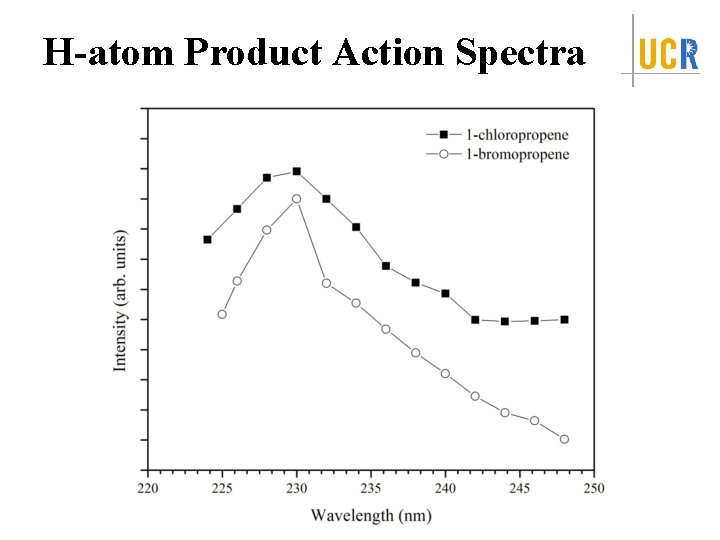
H-atom Product Action Spectra

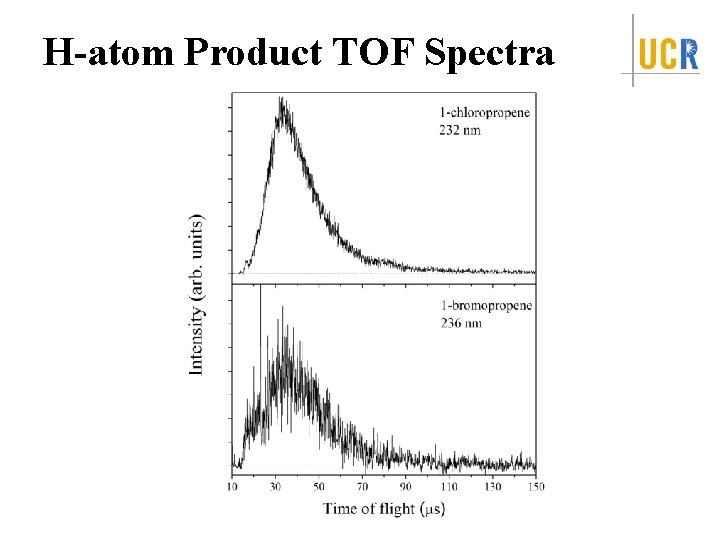
H-atom Product TOF Spectra

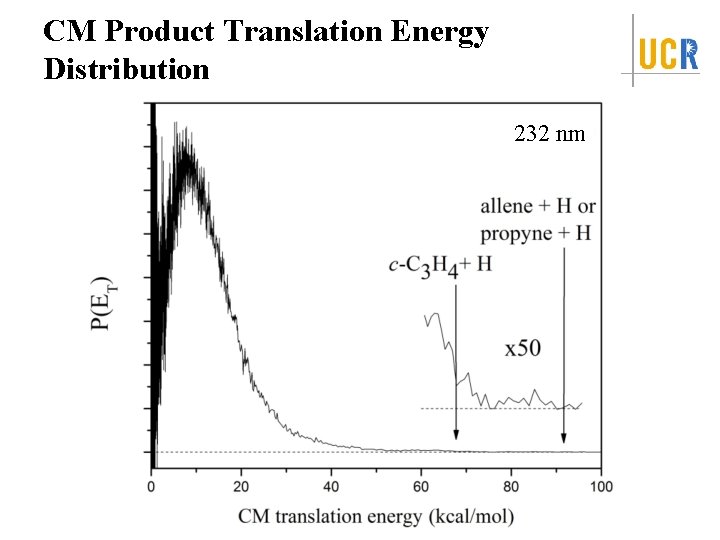
CM Product Translation Energy Distribution 232 nm

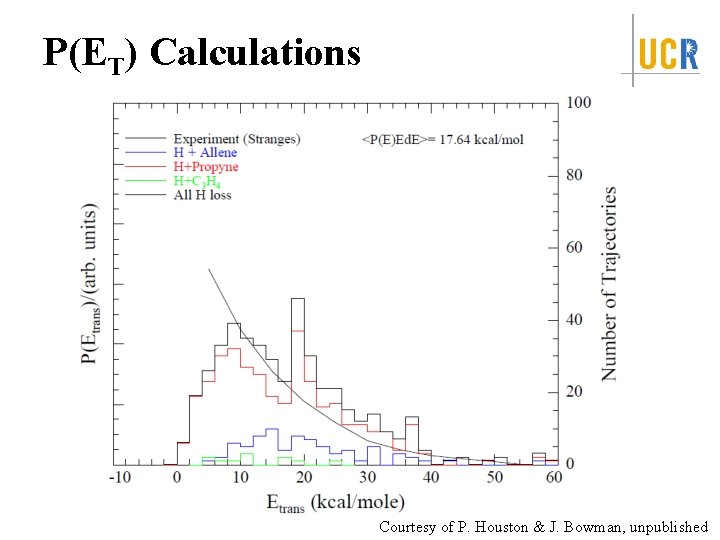
P(ET) Calculations Courtesy of P. Houston & J. Bowman, unpublished

Potential Energy Diagram of C 3 H 5 C. L. Currie et al. J. Chem. Phys. 1966, 45, 488 M. Gasser et al. J. Phys. Chem. A 2010, 114, 4704 H. J. Deyerl et al. J. Chem. Phys. 1999, 110, 1450 S. G. Davis et al. J. Phys. Chem. A 1999, 103, 5889

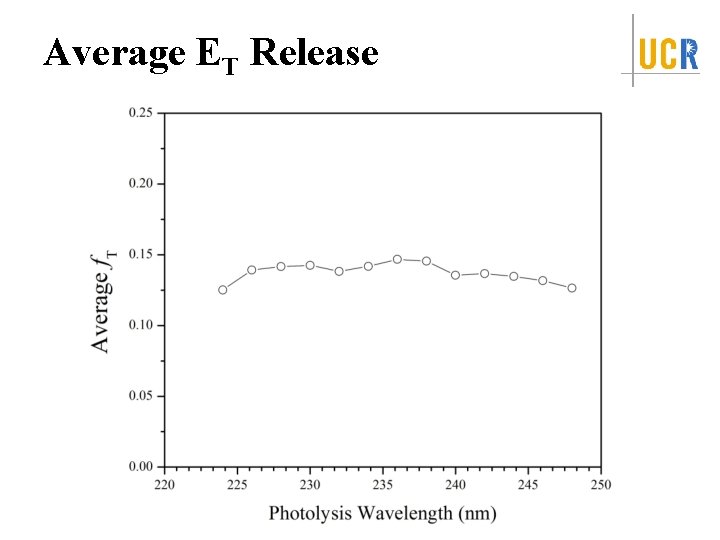
Average ET Release

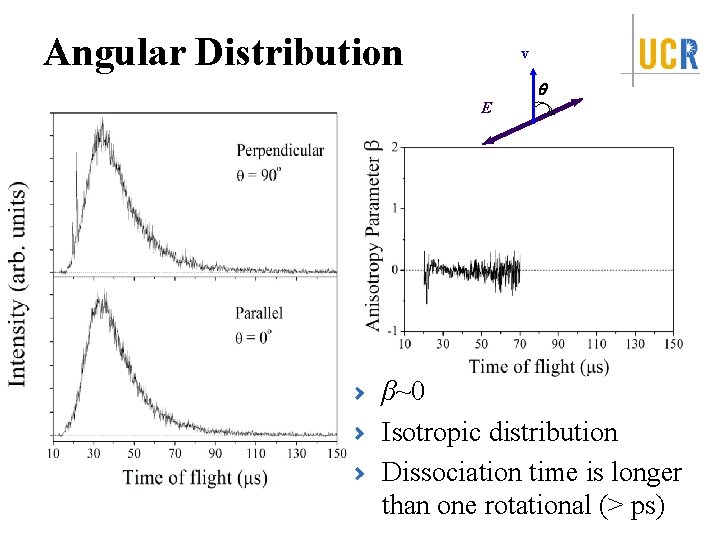
Angular Distribution v q E β~0 Isotropic distribution Dissociation time is longer than one rotational (> ps)

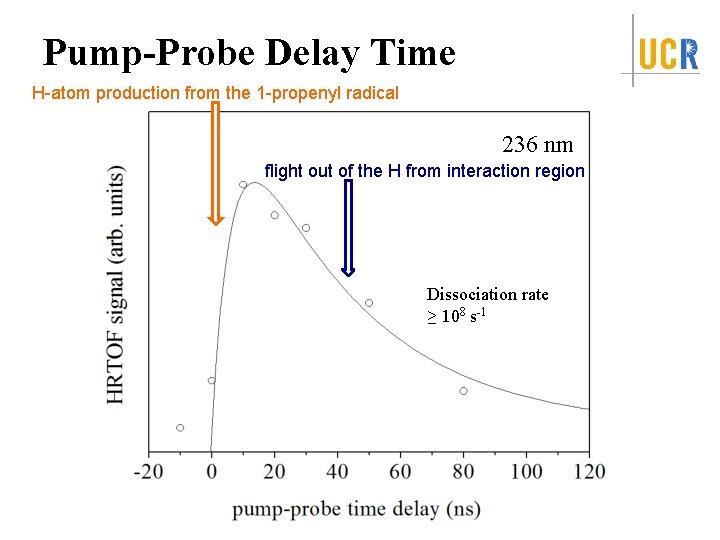
Pump-Probe Delay Time H-atom production from the 1 -propenyl radical 236 nm flight out of the H from interaction region Dissociation rate ≥ 108 s-1

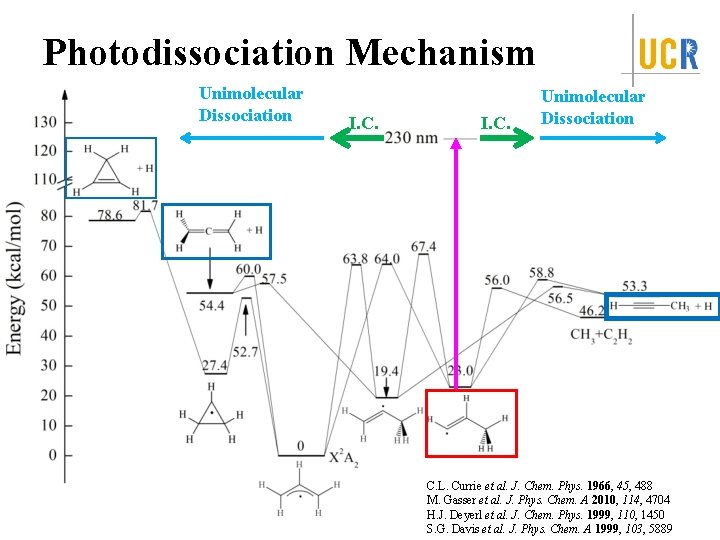
Photodissociation Mechanism Unimolecular Dissociation I. C. Unimolecular Dissociation C. L. Currie et al. J. Chem. Phys. 1966, 45, 488 M. Gasser et al. J. Phys. Chem. A 2010, 114, 4704 H. J. Deyerl et al. J. Chem. Phys. 1999, 110, 1450 S. G. Davis et al. J. Phys. Chem. A 1999, 103, 5889

Summary Identify the first UV absorption feature in the action spectra <f. T> = 0. 125 -0. 14 Isotropic angular distribution, β ~ 0 Dissociation time: ps < t ≤ 10 ns Dissociation Mechanism: internal conversion from excited electronic state to ground electronic state followed by unimolecular dissociation on ground state

Acknowledgements Dr. Jingsong Zhang Dr. Yu Song, UC Davis Jessy Lemieux Lydia Plett Paul Jones Mixtli Campos-Pineda Dr. Christopher Brazier, California State University, Long Beach Trajectory calculations Dr. Paul Houston, Georgia Tech Dr. Joel Bowman, Emory University Funding NSF
 Propenyl radical
Propenyl radical Misfolded
Misfolded Unit 6 radical functions homework 8
Unit 6 radical functions homework 8 What is an entire radical
What is an entire radical Entire to mixed radical
Entire to mixed radical 6-2 multiplying and dividing radical expressions
6-2 multiplying and dividing radical expressions Harrison bergeron theme statement
Harrison bergeron theme statement Scoatere de sub radical calculator
Scoatere de sub radical calculator Simplifying radicals quiz
Simplifying radicals quiz Radical republicans
Radical republicans Romantic radical design
Romantic radical design Radical reconstruction begins
Radical reconstruction begins Bpo 개시 온도
Bpo 개시 온도 11-9 practice b solving radical equations
11-9 practice b solving radical equations Radical republicans def
Radical republicans def Radical do verbo voar
Radical do verbo voar Rational and radical equations
Rational and radical equations Verbos de cambio radical
Verbos de cambio radical