Treatment of Mast Cell Activation Syndrome in Patients











- Slides: 43

Treatment of Mast Cell Activation Syndrome in Patients with Postural Orthostatic Tachycardia Syndrome and Hereditary Connective Tissue Disorders PETER VADAS MD, PHD, FRCPC DIRECTOR DIVISION OF ALLERGY AND CLINICAL IMMUNOLOGY ST. MICHAEL’S HOSPITAL UNIVERSITY OF TORONTO

Mast Cell

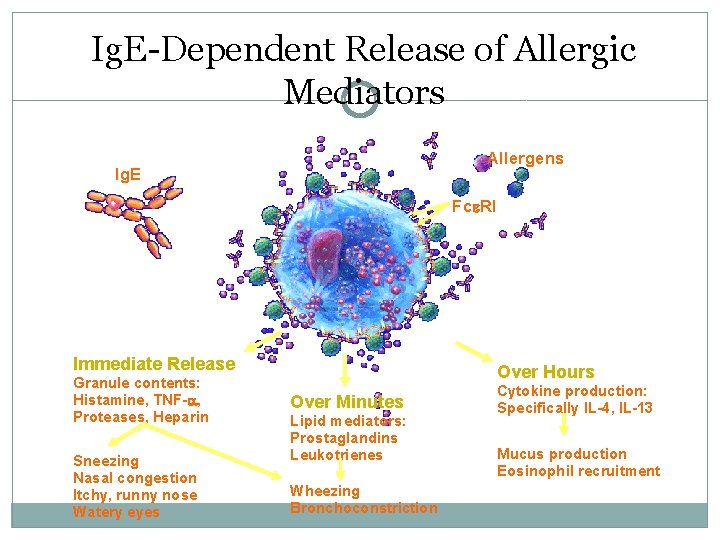
Ig. E-Dependent Release of Allergic Mediators Allergens Ig. E Fc RI Immediate Release Granule contents: Histamine, TNF- , Proteases, Heparin Sneezing Nasal congestion Itchy, runny nose Watery eyes Over Hours Over Minutes Lipid mediators: Prostaglandins Leukotrienes Wheezing Bronchoconstriction Cytokine production: Specifically IL-4, IL-13 Mucus production Eosinophil recruitment


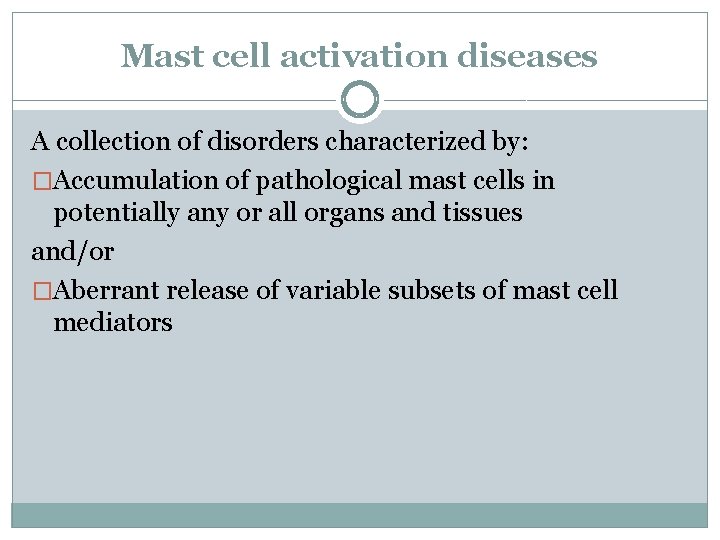
Mast cell activation diseases A collection of disorders characterized by: �Accumulation of pathological mast cells in potentially any or all organs and tissues and/or �Aberrant release of variable subsets of mast cell mediators

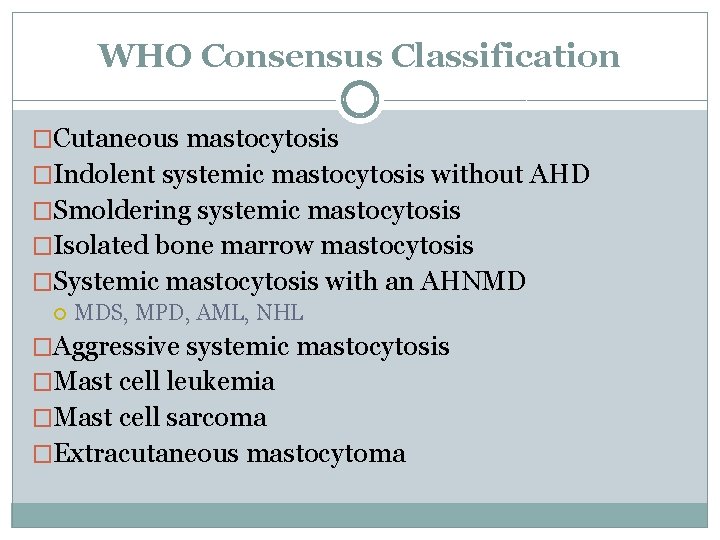
WHO Consensus Classification �Cutaneous mastocytosis �Indolent systemic mastocytosis without AHD �Smoldering systemic mastocytosis �Isolated bone marrow mastocytosis �Systemic mastocytosis with an AHNMD MDS, MPD, AML, NHL �Aggressive systemic mastocytosis �Mast cell leukemia �Mast cell sarcoma �Extracutaneous mastocytoma

Mast Cell Activation Diseases �Mast cell activation syndrome (MCAS)

Clinical Presentation of MCAS �Very diverse! �Due to widespread distribution of mast cells Great heterogeneity of aberrant mediator expression patterns �Symptoms can occur in virtually all organs and tissues �Symptoms can occur in an erratic staggered manner �Wax and wane over years to decades

Signs and Symptoms of Mast Cell Disorders �Skin: episodic flushing, itching, hives �CVS: episodic tachycardia, hypotension, lightheadedness �GI: cramps, diarrhea, heartburn, nausea, vomiting, peptic ulcer, hepatomegaly, splenomegaly, elevated hepatic transaminases, ascites �MSK: osteoporosis, diffuse soft tissue pain

Signs and symptoms cont’d �Constitutional: fatigue, headache �Respiratory: asthma-like symptoms, dyspnea �Neuropsychiatric: headache, neuropathic pain, polyneuropathy, difficulty concentrating, anxiety, forgetfulness, vertigo, lightheadedness, tinnitus

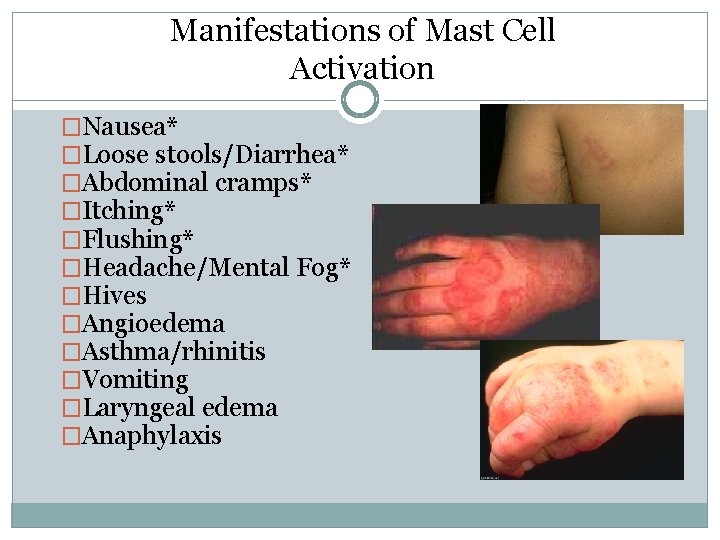
Manifestations of Mast Cell Activation �Nausea* �Loose stools/Diarrhea* �Abdominal cramps* �Itching* �Flushing* �Headache/Mental Fog* �Hives �Angioedema �Asthma/rhinitis �Vomiting �Laryngeal edema �Anaphylaxis

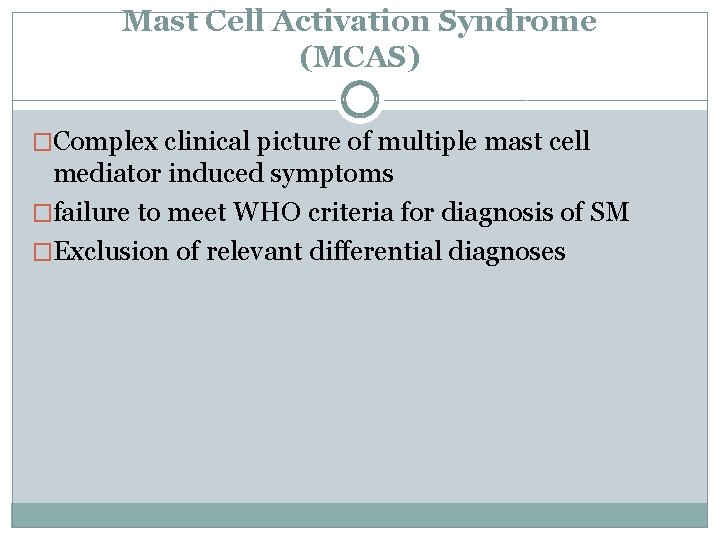
Mast Cell Activation Syndrome (MCAS) �Complex clinical picture of multiple mast cell mediator induced symptoms �failure to meet WHO criteria for diagnosis of SM �Exclusion of relevant differential diagnoses

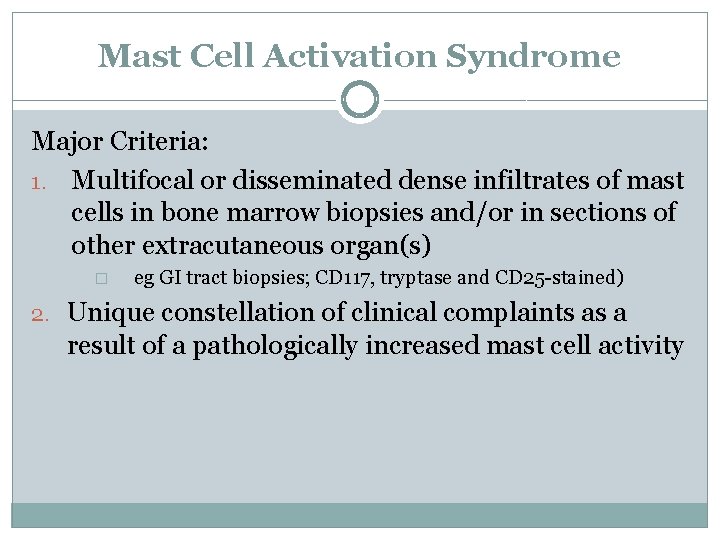
Mast Cell Activation Syndrome Major Criteria: 1. Multifocal or disseminated dense infiltrates of mast cells in bone marrow biopsies and/or in sections of other extracutaneous organ(s) � eg GI tract biopsies; CD 117, tryptase and CD 25 -stained) 2. Unique constellation of clinical complaints as a result of a pathologically increased mast cell activity

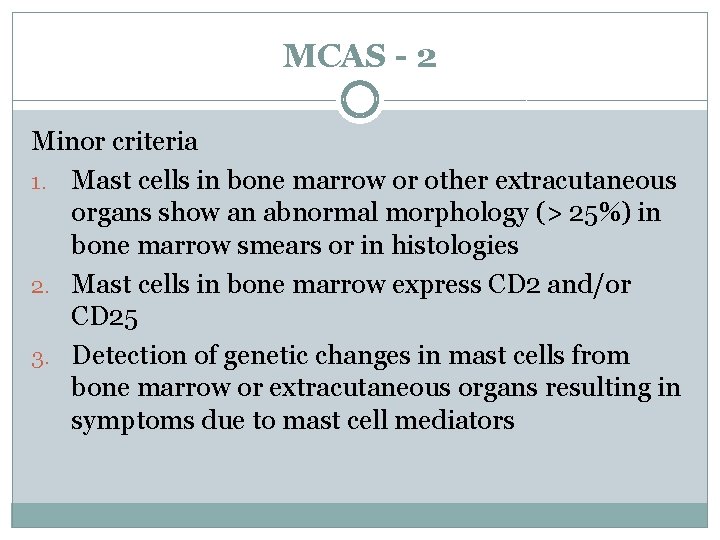
MCAS - 2 Minor criteria 1. Mast cells in bone marrow or other extracutaneous organs show an abnormal morphology (> 25%) in bone marrow smears or in histologies 2. Mast cells in bone marrow express CD 2 and/or CD 25 3. Detection of genetic changes in mast cells from bone marrow or extracutaneous organs resulting in symptoms due to mast cell mediators

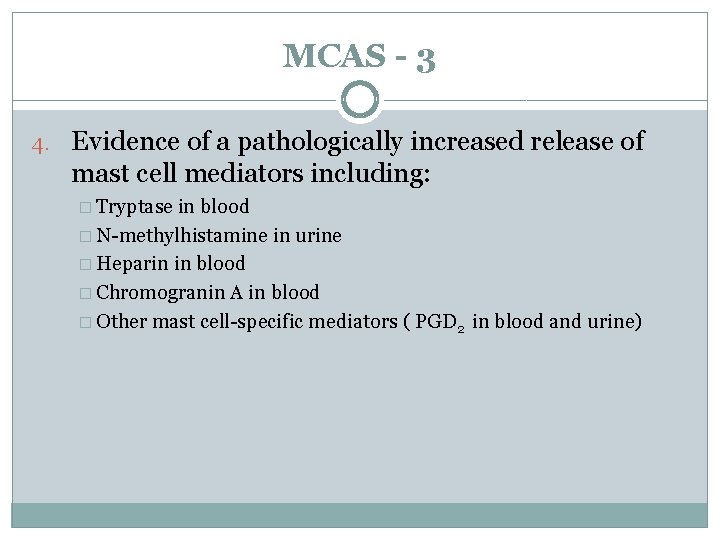
MCAS - 3 4. Evidence of a pathologically increased release of mast cell mediators including: � Tryptase in blood � N-methylhistamine in urine � Heparin in blood � Chromogranin A in blood � Other mast cell-specific mediators ( PGD 2 in blood and urine)

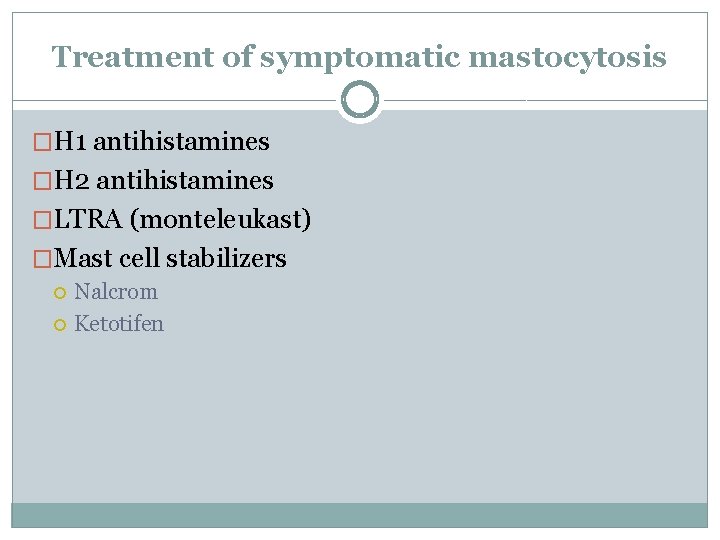
Treatment of symptomatic mastocytosis �H 1 antihistamines �H 2 antihistamines �LTRA (monteleukast) �Mast cell stabilizers Nalcrom Ketotifen

Experimental treatment of MCAS �Thalidomide �Plaquenil �Omalizumab

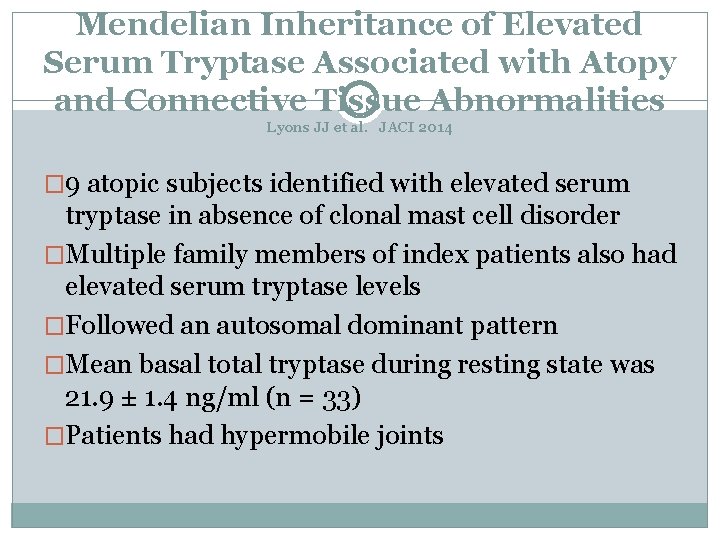
Mendelian Inheritance of Elevated Serum Tryptase Associated with Atopy and Connective Tissue Abnormalities Lyons JJ et al. JACI 2014 � 9 atopic subjects identified with elevated serum tryptase in absence of clonal mast cell disorder �Multiple family members of index patients also had elevated serum tryptase levels �Followed an autosomal dominant pattern �Mean basal total tryptase during resting state was 21. 9 ± 1. 4 ng/ml (n = 33) �Patients had hypermobile joints

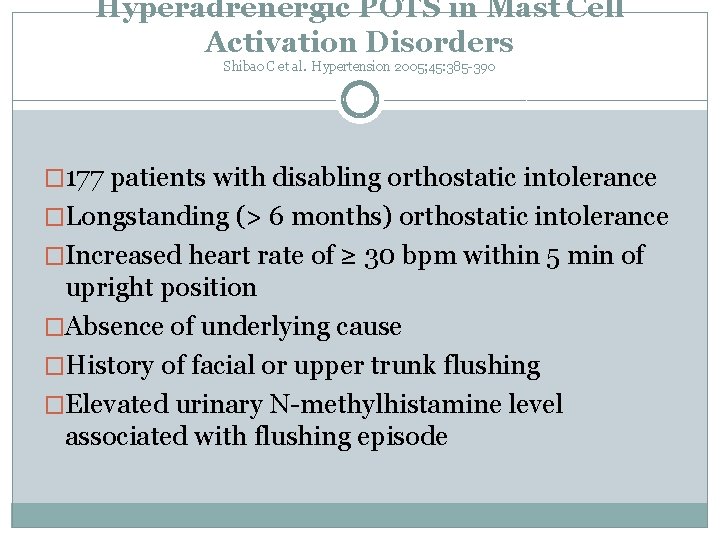
Hyperadrenergic POTS in Mast Cell Activation Disorders Shibao C et al. Hypertension 2005; 45: 385 -390 � 177 patients with disabling orthostatic intolerance �Longstanding (> 6 months) orthostatic intolerance �Increased heart rate of ≥ 30 bpm within 5 min of upright position �Absence of underlying cause �History of facial or upper trunk flushing �Elevated urinary N-methylhistamine level associated with flushing episode

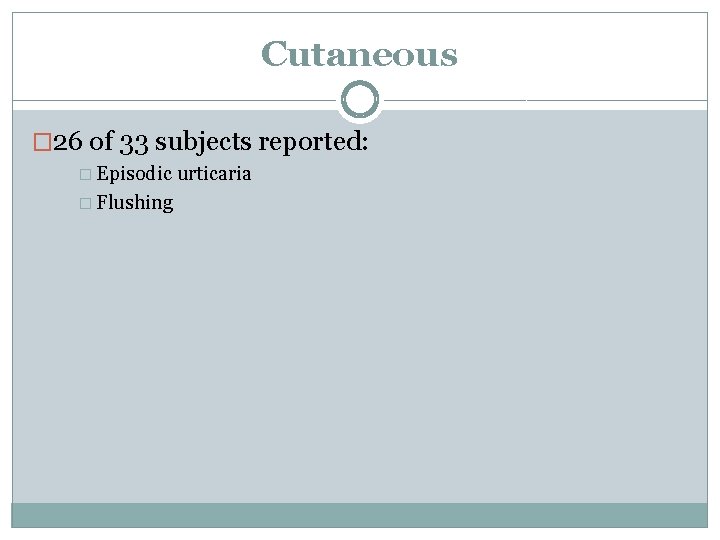
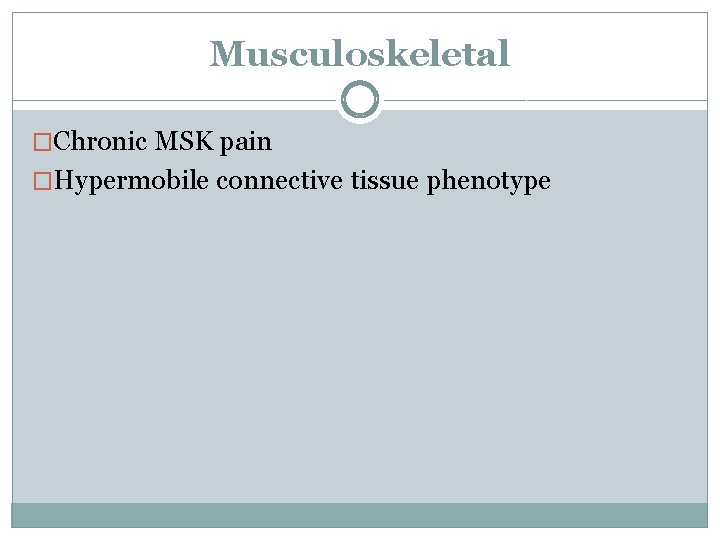
Clinical Characteristics � 5 clusters of clinical characteristics � Cutaneous � Connective tissue � Gastrointestinal � Atopic � Neuropsychiatric

Cutaneous � 26 of 33 subjects reported: � Episodic � Flushing urticaria

Gastrointestinal �Crampy abdominal pain �Urgency diarrhea �Gastroesophageal reflux �IBS-like symptoms �Eosinophilic esophagitis

Atopic � 31 or 33 subjects had atopic symptoms incl: � Environmental � Asthma allergies

Musculoskeletal �Chronic MSK pain �Hypermobile connective tissue phenotype

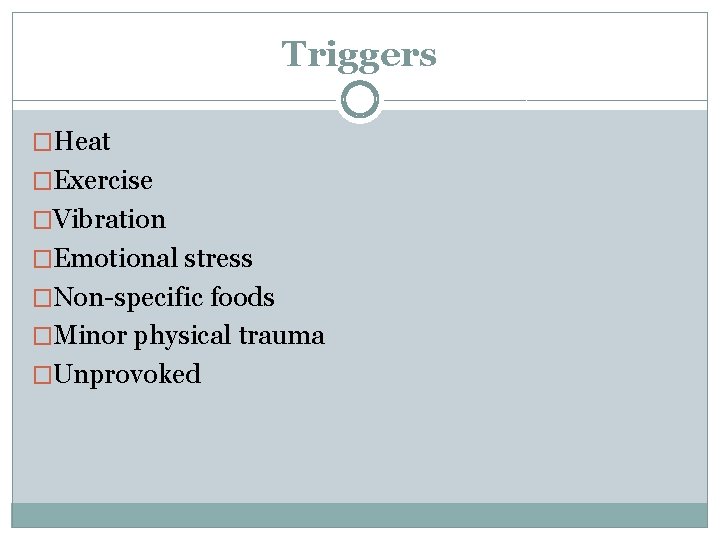
Triggers �Heat �Exercise �Vibration �Emotional stress �Non-specific foods �Minor physical trauma �Unprovoked

�None of subjects met WHO criteria for either systemic mastocytosis or monoclonal mast cell activation �Mast cell aggregates not present �No aberrant expression of CD 2/CD 25 �KIT D 816 V mutation absent �Spindle shaped mast cells seen in only 1 patient

Co-segregation of POTS, EDS and MCAS Cheung I and Vadas P JACI 2014 �Patients with POTS and hypermobility often describe symptoms of mast cell activation �Participants with diagnoses of POTS and EDS were recruited from throughout North America through an online support group

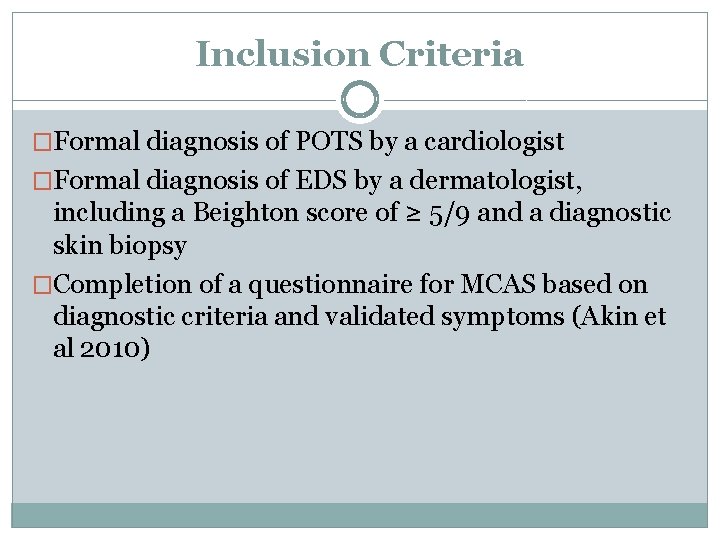
Inclusion Criteria �Formal diagnosis of POTS by a cardiologist �Formal diagnosis of EDS by a dermatologist, including a Beighton score of ≥ 5/9 and a diagnostic skin biopsy �Completion of a questionnaire for MCAS based on diagnostic criteria and validated symptoms (Akin et al 2010)

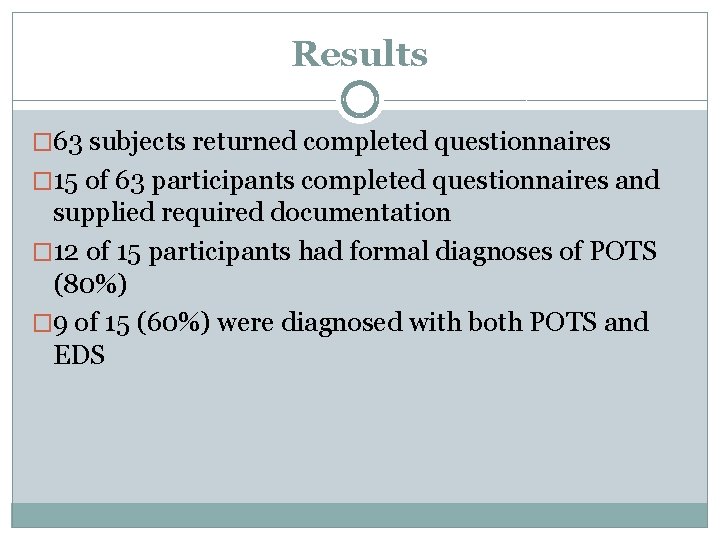
Results � 63 subjects returned completed questionnaires � 15 of 63 participants completed questionnaires and supplied required documentation � 12 of 15 participants had formal diagnoses of POTS (80%) � 9 of 15 (60%) were diagnosed with both POTS and EDS

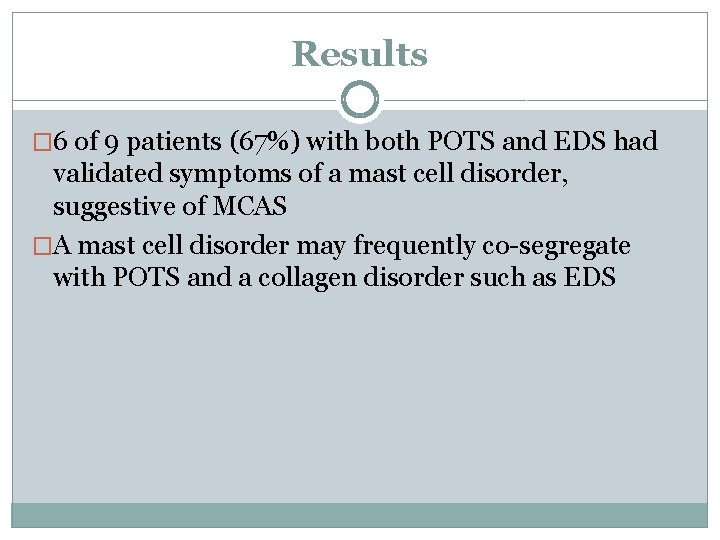
Results � 6 of 9 patients (67%) with both POTS and EDS had validated symptoms of a mast cell disorder, suggestive of MCAS �A mast cell disorder may frequently co-segregate with POTS and a collagen disorder such as EDS

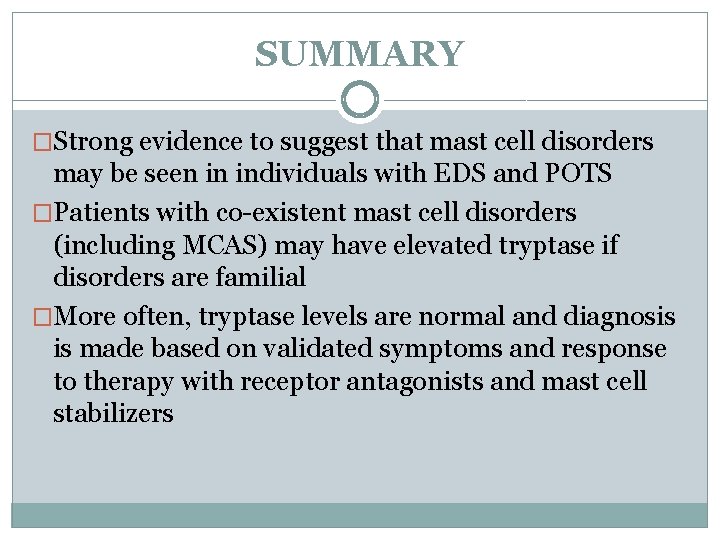
SUMMARY �Strong evidence to suggest that mast cell disorders may be seen in individuals with EDS and POTS �Patients with co-existent mast cell disorders (including MCAS) may have elevated tryptase if disorders are familial �More often, tryptase levels are normal and diagnosis is made based on validated symptoms and response to therapy with receptor antagonists and mast cell stabilizers

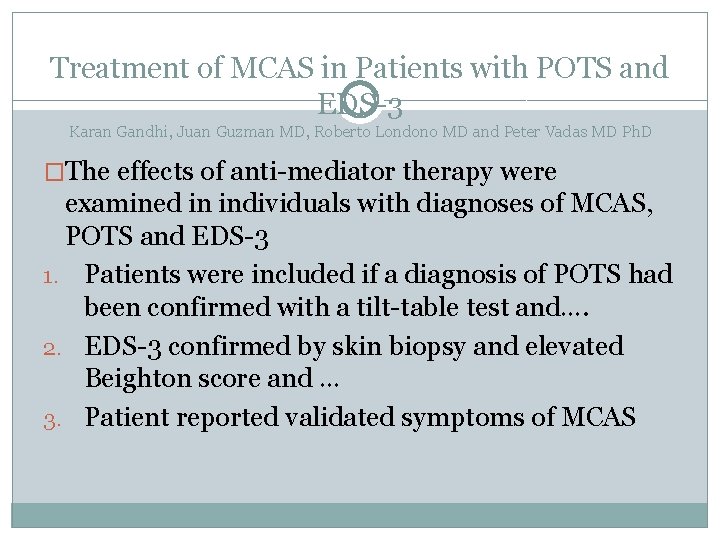
Treatment of MCAS in Patients with POTS and EDS-3 Karan Gandhi, Juan Guzman MD, Roberto Londono MD and Peter Vadas MD Ph. D �The effects of anti-mediator therapy were examined in individuals with diagnoses of MCAS, POTS and EDS-3 1. Patients were included if a diagnosis of POTS had been confirmed with a tilt-table test and…. 2. EDS-3 confirmed by skin biopsy and elevated Beighton score and … 3. Patient reported validated symptoms of MCAS

�Response to treatment was assessed based on self- reported changes in symptoms

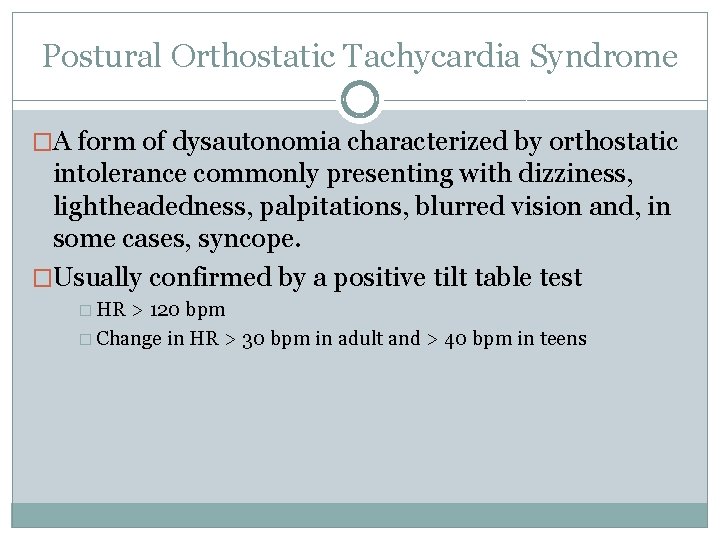
Postural Orthostatic Tachycardia Syndrome �A form of dysautonomia characterized by orthostatic intolerance commonly presenting with dizziness, lightheadedness, palpitations, blurred vision and, in some cases, syncope. �Usually confirmed by a positive tilt table test � HR > 120 bpm � Change in HR > 30 bpm in adult and > 40 bpm in teens

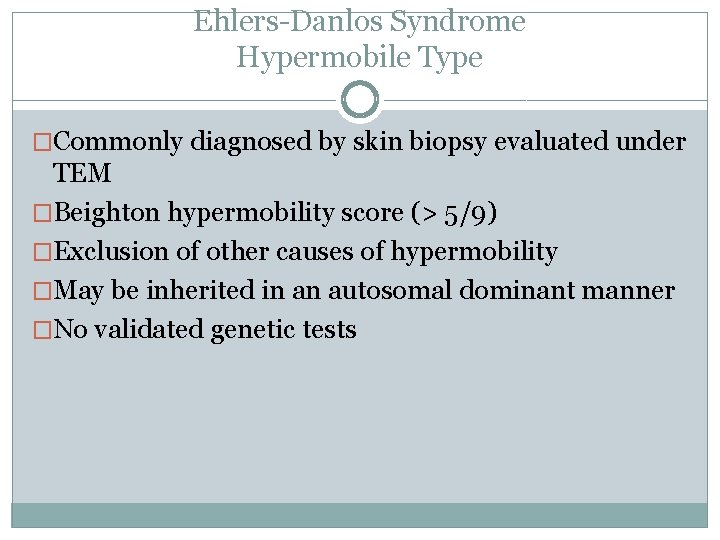
Ehlers-Danlos Syndrome Hypermobile Type �Commonly diagnosed by skin biopsy evaluated under TEM �Beighton hypermobility score (> 5/9) �Exclusion of other causes of hypermobility �May be inherited in an autosomal dominant manner �No validated genetic tests

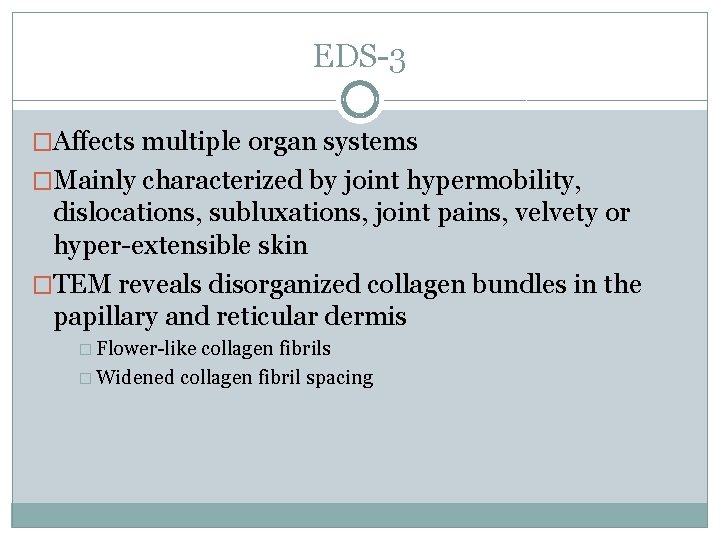
EDS-3 �Affects multiple organ systems �Mainly characterized by joint hypermobility, dislocations, subluxations, joint pains, velvety or hyper-extensible skin �TEM reveals disorganized collagen bundles in the papillary and reticular dermis � Flower-like collagen fibrils � Widened collagen fibril spacing

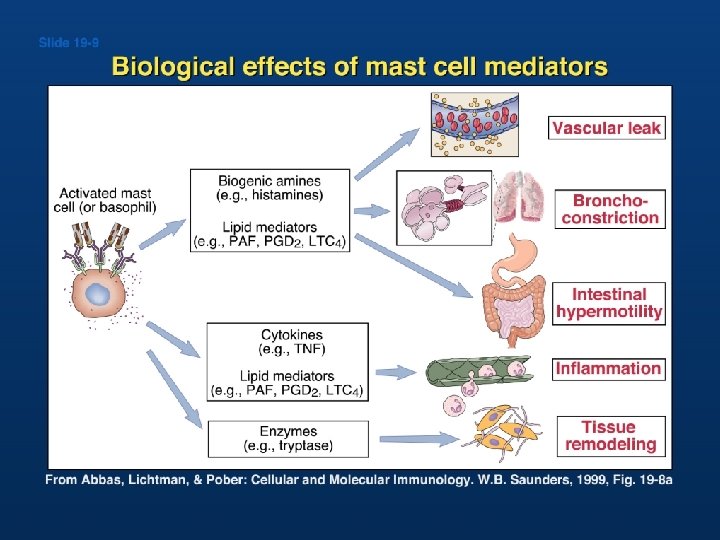
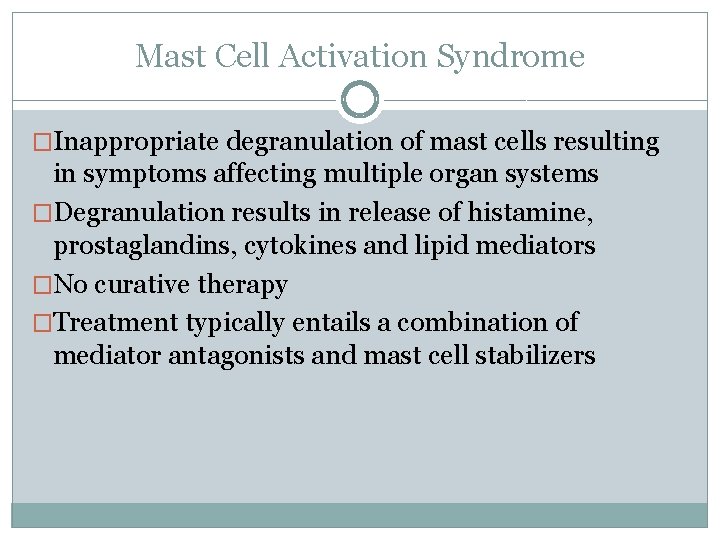
Mast Cell Activation Syndrome �Inappropriate degranulation of mast cells resulting in symptoms affecting multiple organ systems �Degranulation results in release of histamine, prostaglandins, cytokines and lipid mediators �No curative therapy �Treatment typically entails a combination of mediator antagonists and mast cell stabilizers

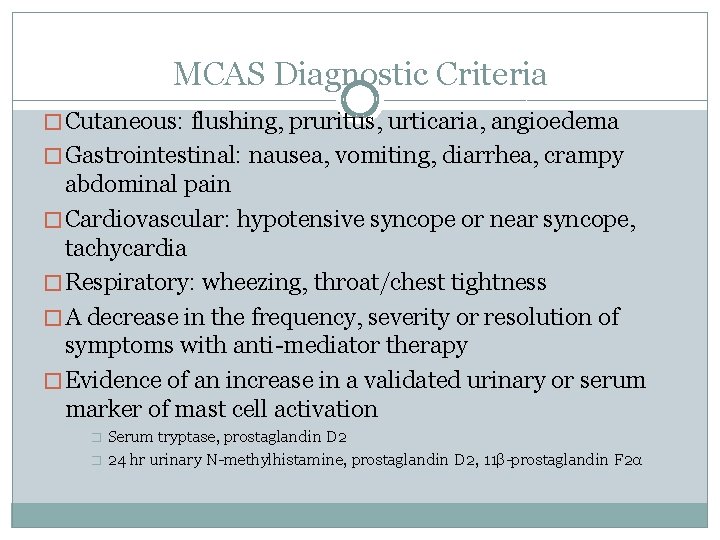
MCAS Diagnostic Criteria � Cutaneous: flushing, pruritus, urticaria, angioedema � Gastrointestinal: nausea, vomiting, diarrhea, crampy abdominal pain � Cardiovascular: hypotensive syncope or near syncope, tachycardia � Respiratory: wheezing, throat/chest tightness � A decrease in the frequency, severity or resolution of symptoms with anti-mediator therapy � Evidence of an increase in a validated urinary or serum marker of mast cell activation � � Serum tryptase, prostaglandin D 2 24 hr urinary N-methylhistamine, prostaglandin D 2, 11β-prostaglandin F 2α



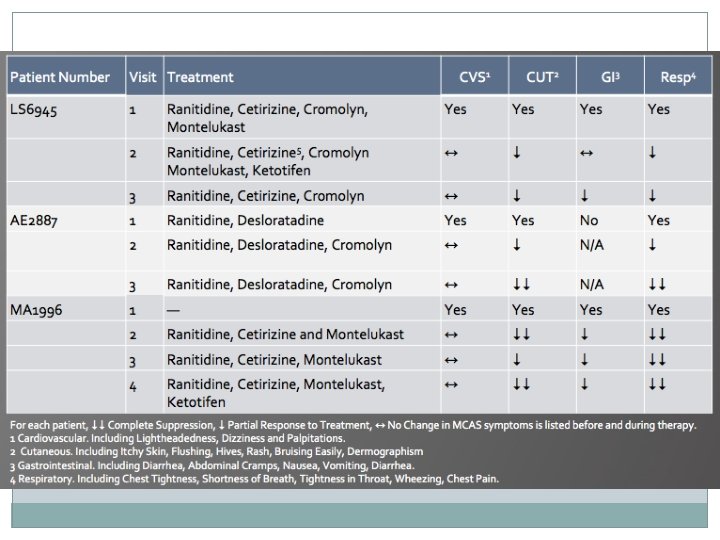
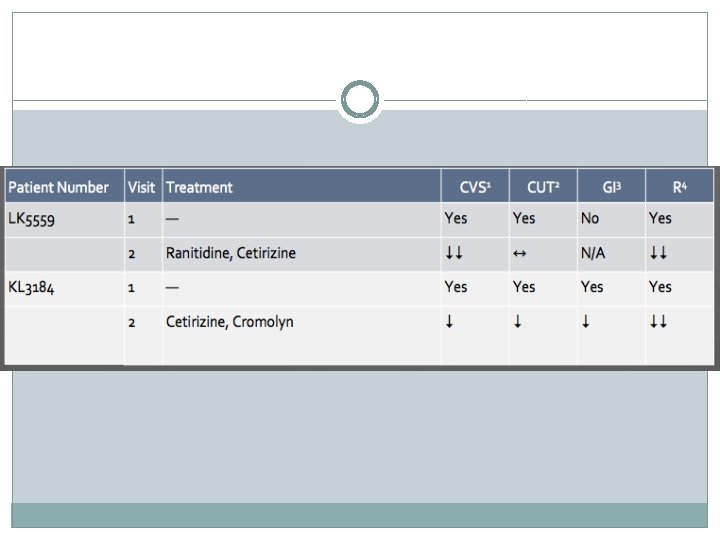
Discussion �Anti-mediator therapy led to self-reported improvement in � cutaneous (4/5) � gastrointestinal (3/3) � respiratory (5/5) �All patients reported at least some positive response to therapy �Only 2/5 patients reported a positive response to cardiovascular symptoms

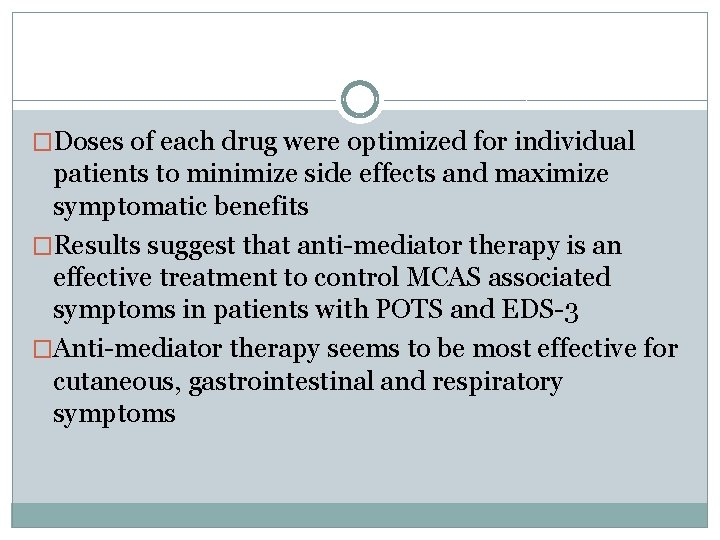
�Doses of each drug were optimized for individual patients to minimize side effects and maximize symptomatic benefits �Results suggest that anti-mediator therapy is an effective treatment to control MCAS associated symptoms in patients with POTS and EDS-3 �Anti-mediator therapy seems to be most effective for cutaneous, gastrointestinal and respiratory symptoms

�Karan Gandhi �Ingrid Cheung �Juan Guzman MD �Roberto Mendoza MD �Scott Walsh MD
 Mast cell activation syndrome
Mast cell activation syndrome Pots symptoms
Pots symptoms Jacking cage for tower crane
Jacking cage for tower crane Activation tree and activation record
Activation tree and activation record Sertoli cell only syndrome treatment
Sertoli cell only syndrome treatment Theophylline mechanism of action
Theophylline mechanism of action Tiotropium mechanism of action
Tiotropium mechanism of action Vkc vs akc
Vkc vs akc Classification of antitussive
Classification of antitussive Mast cell stabilizer
Mast cell stabilizer Dr leonard weinstock mast cell
Dr leonard weinstock mast cell Mast cell tumor german shorthaired pointer
Mast cell tumor german shorthaired pointer Mast cell progenitor
Mast cell progenitor Mast cell progenitor
Mast cell progenitor Bryostatin
Bryostatin Intraembryonic mesoderm
Intraembryonic mesoderm Hsv2
Hsv2 Cascade induction
Cascade induction Cva
Cva Nk cell activation markers
Nk cell activation markers Life expectancy of sickle cell patients
Life expectancy of sickle cell patients Mirizzi syndrome types
Mirizzi syndrome types Boerhaave's syndrome
Boerhaave's syndrome Amanda morra
Amanda morra Stem cell therapy rhode island
Stem cell therapy rhode island Plasma cell mastitis treatment
Plasma cell mastitis treatment Svinjska mast
Svinjska mast Mco 1700
Mco 1700 Mast bumping helicopter
Mast bumping helicopter Patrick rufin
Patrick rufin Svinjska mast cena dis
Svinjska mast cena dis špek za topljenje
špek za topljenje Naval terms and phraseologies
Naval terms and phraseologies Toyota forklift year by serial number
Toyota forklift year by serial number Mast tlif
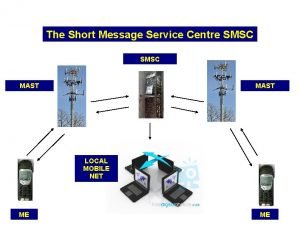
Mast tlif Short message service centre
Short message service centre Sextant medtronic
Sextant medtronic Mast ski team
Mast ski team Uzeglost masti i ulja
Uzeglost masti i ulja Anestezin mast
Anestezin mast Mir camar uddin mast
Mir camar uddin mast Mast tlif
Mast tlif Activation synthesis theory
Activation synthesis theory Electrogravimetry example
Electrogravimetry example