Thoughts on Ethical Pitfalls in Clinical Research Prof








































- Slides: 41

Thoughts on Ethical Pitfalls in Clinical Research Prof Marc Blockman Chair: FHS HREC June 2018 Conflicts of interest: Lots

Research Ethics Committees Burden or Benefit to Research ?

Ethical principles and research

Introduction What is ethics? 2 fundamental objectives of ethics: ● How we ought to act in a given situation ● Provide us with strong reasons for doing so involves a critical reflection of morality with its intent to safeguard human dignity and to promote justice, equality, truth, and trust.

Research Ethics Means of ensuring vulnerable people are protected from exploitation and other forms of harms Evolving language : passive subject → active participant

CHALLENGES CAN THE DEMANDS AND GOALS OF SCIENCE BE PURSUED WITH FULL PROTECTIONS OF THE RIGHTS AND DIGNITY OF THE RESEARCH PARTICIPANT?

SUCCESSES NOT WITHOUT COST

POSNER’S TESTIMONY • “In the workroom next to the dissecting room, fourteen Gypsy twins were waiting and crying bitterly. Dr Mengele didn’t say a word to us, and prepared a 10 cc and a 5 cc syringe. From a box he took Evipal and from another box he took chloroform, and put these on the operating table. After that the first twin was brought in … a 14 year old girl. Dr Mengele ordered me to undress the child and put her head on the dissecting table. Then he injected the Evipal into her right arm intravenously. After the child had fallen asleep, he felt for the left ventricle of the heart and injected 10 cc of chloroform …. After 1 little twitch the child was dead … in this manner all 14 were killed. ”

POSNER’S TESTIMONY Mengele then removed the eyes from the dead twins and shipped them off to Berlin for further study.

Some Pertinent International Instruments & Guidelines • Nuremberg Code – 1947 • Universal Declaration of Human Rights – 1948: “No one shall be subject to torture or to cruel, inhuman or degrading treatment or punishment. In particular, no one shall be subjected without his free consent to medical or scientific experimentation” • Declaration of Helsinki – 1964 (updated x 6 Presently 2013) • Council of International Organisation of Medical Scientists (CIOMS)

Declaration of Helsinki “The design and performance of each experimental procedure involving human subjects should be clearly formulated in an experimental protocol. This protocol should be submitted for consideration, comment, guidance, and where appropriate, approval to a specially appointed ethical review committee, which must be independent of the investigator, the sponsor or any other kind of undue influence. This independent committee should be in conformity with the laws and regulations of the country in which the research experiment is performed. The committee has the right to monitor ongoing trials. The researcher has the obligation to provide monitoring information to the committee, especially any serious adverse events. The researcher should also submit to the committee, for review, information regarding funding, sponsors, institutional affiliations, other potential conflicts of interest and incentives for subjects”.

South African Guidelines • Ethics in Health Research: Principles, Structures & Processes – 2 nd ED 2015 • Guidelines for Good Practice in the Conduct of Clinical Trials with Human Participants in South Africa – 2006 (2 ed) • Ethical and Legal Guidelines for Biotechnology Research in SA (DST) - 2006 • Health Professions Council of South Africa Ethical Guidelines • National Health Act • Bill of Rights of the Constitution of South Africa

Principle-based Ethics • • Autonomy Beneficence Non-maleficence Justice Lack hierarchical order rendering ranking arbitrary

Respect for Persons • Respect for autonomy - those capable of deliberation about personal choices to be treated with respect for their capacity of selfdetermination • Protection of persons with diminished / impaired autonomy – dependant / vulnerable to be afforded security against harm /abuse

Justice • Ethical obligation to treat each person in accordance with what is right & proper • In research: • primarily distributive justice – equitable distribution of both burdens & benefits of research participation • To be responsive to health needs & priorities – intervention to be reasonably available • Study should leave participant / community better off / no worse off

Situation in South Africa • home to a large number of vulnerable groups of poor populations - potential for misuse of power cannot be ignored • Costs are lower than in many other countries with similar standards, less chance of litigation • SA viewed as a valuable “gateway” for launching clinical research efforts northwards into the rest of Africa

Ethical Review 1. A South African-based research ethics committee must review the ethical and scientific rigour of all research conducted in South Africa. 2. No research involving humans can begin until the ethics committee has granted approval. 3. Researchers must obtain ongoing approval, at least annually, throughout the research activity.

Ethics committees • Independent committee consisting of mixture of experts and lay members • Neither the researchers nor sponsors should ever be the sole judges of whether their projects conform to accepted guidelines • Objectives: to protect the subjects & provide public reassurance

Ethics committees • NOTE: • Approval by an ethics committee also protects the investigators and the institutions where such trials are conducted

REC looks at: • 1. Whether the scientific quality of the protocol has been properly assessed – any trial which is unscientific is also unethical • 2. Whether investigator & any other persons who will be involved in the conduct of the trial are competent & the facilities adequate • 3. Possible hazards to trial subjects, & precautions taken to deal with them

REC looks at: • 4. Appropriate informed consent • 5. Adequate compensation arrangements in place in the event of medicine-induced injury, or other damage to the subject arising from the conduct of the trial • 6. Methods of recruitment, details of information given out to subjects, any payments made to them • 7. Payments to the investigator site

REC Review • Primary role – protection of rights & welfare of research participants • Provide ethical advice to researchers to assist decisionmaking on adequacy of proposed research projects regarding participant protection

REC CHALLENGES

Defining the Therapeutic Misconception “Therapeutic misconception exists when individuals do not understand that the defining purpose of clinical research is to produce generalizable knowledge, regardless of whether the subjects enrolled in the trial may potentially benefit from the intervention under study or from other aspects of the clinical trial “ Henderson, et al. Clinical Trials and Medical Care: Defining the Therapeutic Misconception. PLo. S Medicine (2007): 4; 1735 -1738

Vulnerable “exposed to being attacked or harmed, either physically or emotionally” (South African Concise Oxford Dictionary – 2015)

Vulnerable Groups - Characteristics • Susceptibility to exploitation • Unequal power relationships • Dependency • Erosion of voluntariness element of consent

Categories • • Patients Those in Institutions Diminished Capacity Economically Disadvantaged Socially Disadvantaged Minority Groups Refugees

CIOMS Ethical Justification for Involving Vulnerable Persons • Couldn’t be carried out as well with non-vulnerable persons • Problem related to vulnerability • Participants and class will have reasonable availability to products • Risks without health benefits will not be greater than routine unless authorized • Supplemental consent when needed

Using Money as a Recruitment Tool – Some Concerns • May lead to undue inducement hence decreasing voluntariness or understanding • May result in economically disadvantaged bearing unduly large share of risks & burdens of research participation • Overuse of participants / communities because of social & administrative availability

Undue Inducement • Takes undue risks & volunteers against better judgment - capacity to exercise free choice undermined at risk of harm

Fair Compensation - CIOMS • Transport • Lost earnings • Other expenses associated with participation • Meal To be viewed in light of particular culture & population

UCT FHS HREC

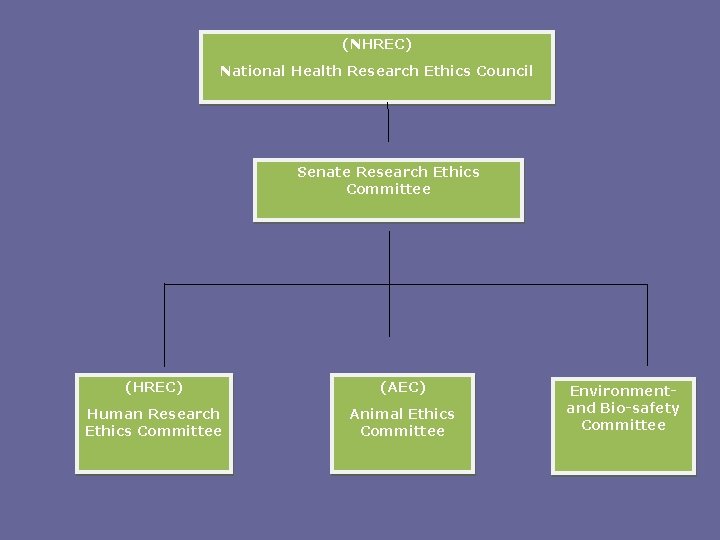
(NHREC) National Health Research Ethics Council Senate Research Ethics Committee (HREC) (AEC) Human Research Ethics Committee Animal Ethics Committee Environmentand Bio-safety Committee

Top Ten Reasons Why Approval of Your REC Application May Be Delayed

Top Ten Reasons for Delays 1) Insufficient information upon which to determine risk/benefit 2) Benefit > Risk not made explicit 3) Study Design Inadequate 4) Informed consent Issues 5) Privacy/confidentiality Issues Not Addressed 6) Selection of Subjects Not Equitable or Described 7) Research and Clinical Care Difficult to Distinguish 8) Unethical Study 9) Administrative Details Not Addressed Appropriately 10) Response format

Do’s and Don’t’s • If not written down the REC doesn’t know it (REC cannot make [best or worst case] assumptions) • All protocol issues need to be in the protocol (i. e. not just in the IC doc) • Avoid cut and paste errors (very common) and checking off the wrong boxes

Do’s and Don’t’s • Avoid typographical errors • Be explicit about why a drug study is exempt from the SAHPRA • Consistency throughout application • Avoid saying what has been approved in the past

Before you apply Things to consider: 1. Use the guidelines/Website 2. Proof read the entire application 3. Use template forms found online 4. Use lay language. Information sheet 5. Information sheets for all audiences (children, teachers, parents etc) 6. Consent 7. Informed decisions. Is there ample time for participants to consider information 8. Allow time for review. Ensure that you have allowed sufficient time for ethical review, e. g. Do you have a deadline?

What Makes Research Ethical? • • Social or scientific value Scientific validity Fair subject selection Favorable risk-benefit ratio Independent review Informed consent Respect for potential and enrolled subjects Collaborative partnership Emanual et al. , JAMA, 283, 2000

“ For the things we have to learn before we do them, we learn by doing them. ” - Aristotle

Thank you Questions?