Thermochemistry The study of heat changes that occur










- Slides: 14

Thermochemistry: The study of heat changes that occur during chemical reactions and physical changes of state

Energy – the ability to do work or produce heat n Exists in 2 forms: n Kinetic energy – energy of motion n Potential energy – energy at rest or energy of position n Heat (q) = the energy that transfers from one object to another because of a temperature difference between them n Heat always flows from a warmer object to a cooler object n

Energy Kinetic energy – in a chemical reaction temperature is the determining factor n The higher the temperature…the faster the particles move…the higher the average kinetic energy n Temperature is a measure of the average kinetic energy n Kelvin scale: 0 K = -273 °C n

Law of Conservation of Energy n Law of Conservation of Energy – Energy is neither created nor destroyed

Heat (q) Heat or energy can be in joules, calories, kilocalories, or kilojoules n The SI unit is the joule n 1 Cal = 1000 cal = 1 kcal n 1 cal = 4. 186 J n 1 kcal = 4186 J n 1 J = 0. 239 cal n

Heat Capacity The amount of heat it takes to change an object’s temperature by 1ºC n Depends on an object’s mass n Ex. A cup of water has a greater heat capacity than a drop of water. n

Specific Heat (C) – the amount of heat required to raise 1 gram of a substance by 1 C n Specific heat is an intensive property, and therefore does not depend on size n Every substance has its own specific heat n Ex. Water = 4. 18 J/(g x ºC) Glass = 0. 50 J/(g x ºC) n

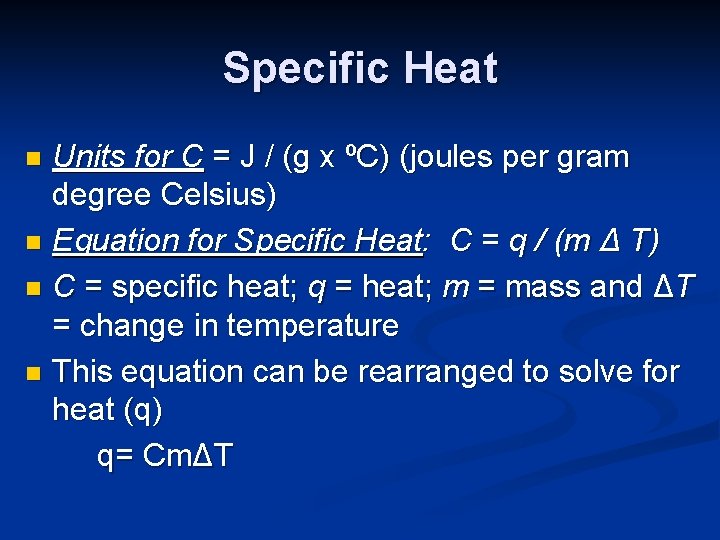
Specific Heat Units for C = J / (g x ºC) (joules per gram degree Celsius) n Equation for Specific Heat: C = q / (m Δ T) n C = specific heat; q = heat; m = mass and ΔT = change in temperature n This equation can be rearranged to solve for heat (q) q= CmΔT n

Specific Heat n A 10. 0 g sample of iron changes temperature from 25. 0 C to 50. 4 C while releasing 114 joules of heat. Calculate the specific heat of iron.

Example C= q/ (m∆T) n C=114 J/ (10. 0 g x 25. 4°C) n C = 0. 45 J/g C n

Another example n If the temperature of 34. 4 g of ethanol increases from 25. 0 C to 78. 8 C how much heat will be absorbed if the specific heat of the ethanol is 2. 44 J/g C

Another example First, rearrange the specific heat formula to solve for heat n q = Cm T n q = (2. 44 J/g°C)(34. 4 g)(78. 8°C - 25°C) n q = 4515. 76 J n

Yet another example n 4. 50 g of a gold nugget absorbs 276 J of heat. What is the final temperature of the gold if the initial temperature was 25. 0 C & the specific heat of the gold is 0. 129 J/g C

Yet another example n n n n C= q/ (m∆T); rearrange to find ∆T = q / (C x m) ∆T = 276 J / (. 129 J/g°C x 4. 50 g) T = 475. 45 C T = Tf-Ti 475. 45 = Tf-25 Tf = 500. 45 C
 Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is study of
Thermochemistry is study of Thermochemistry is concerned with the study of
Thermochemistry is concerned with the study of Thermochemistry is the study of *
Thermochemistry is the study of * Study of energy and its transformations
Study of energy and its transformations Ilmu yang mempelajari tentang kalor reaksi disebut
Ilmu yang mempelajari tentang kalor reaksi disebut Thermochemistry is study of
Thermochemistry is study of Thermochemistry is the study of
Thermochemistry is the study of Phân độ lown
Phân độ lown Block nhĩ thất độ 2 mobitz 2
Block nhĩ thất độ 2 mobitz 2 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều