Thermochemistry 2 Hesss Law Heat of Formation Heat

- Slides: 22

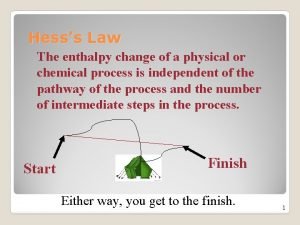
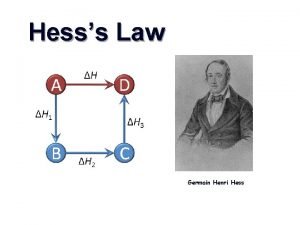
Thermochemistry 2 Hess’s Law Heat of Formation Heat of Combustion Bond Enthalpy

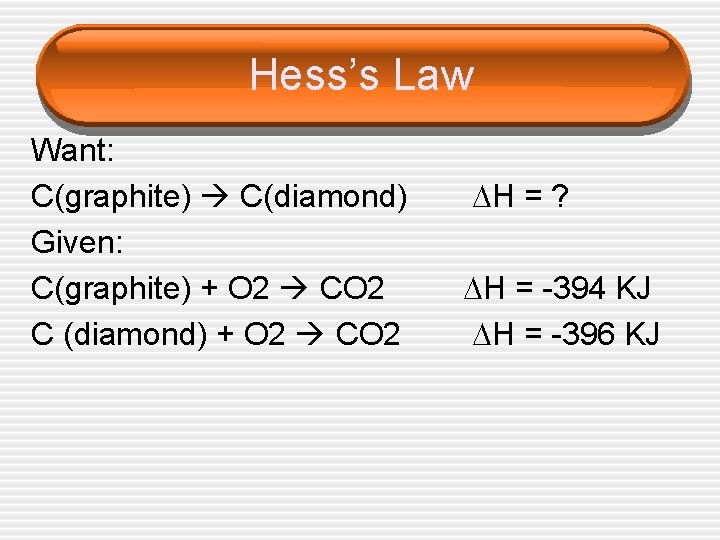
Hess’s Law Want: C(graphite) C(diamond) Given: C(graphite) + O 2 CO 2 C (diamond) + O 2 CO 2 H = ? H = -394 KJ H = -396 KJ

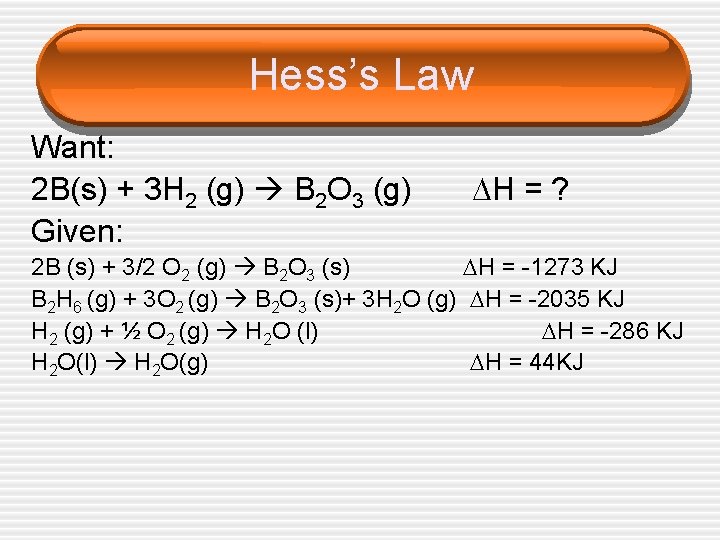
Hess’s Law Want: 2 B(s) + 3 H 2 (g) B 2 O 3 (g) Given: H = ? 2 B (s) + 3/2 O 2 (g) B 2 O 3 (s) H = -1273 KJ B 2 H 6 (g) + 3 O 2 (g) B 2 O 3 (s)+ 3 H 2 O (g) H = -2035 KJ H 2 (g) + ½ O 2 (g) H 2 O (l) H = -286 KJ H 2 O(l) H 2 O(g) H = 44 KJ

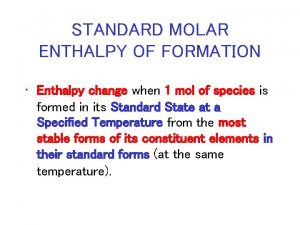
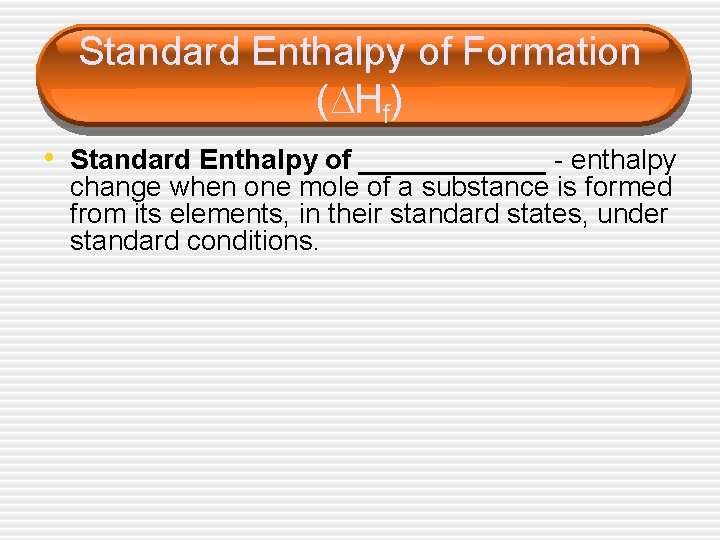
Standard Enthalpy of Formation ( Hf) • Standard Enthalpy of ______ - enthalpy change when one mole of a substance is formed from its elements, in their standard states, under standard conditions.

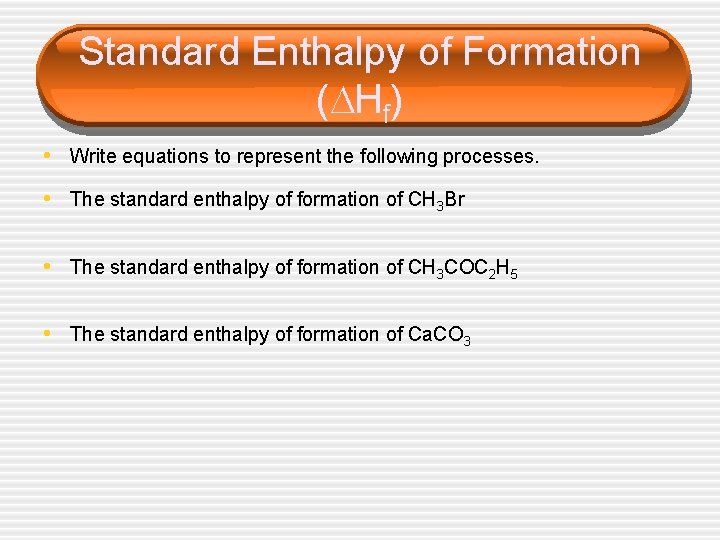
Standard Enthalpy of Formation ( Hf) • Write equations to represent the following processes. • The standard enthalpy of formation of CH 3 Br • The standard enthalpy of formation of CH 3 COC 2 H 5 • The standard enthalpy of formation of Ca. CO 3

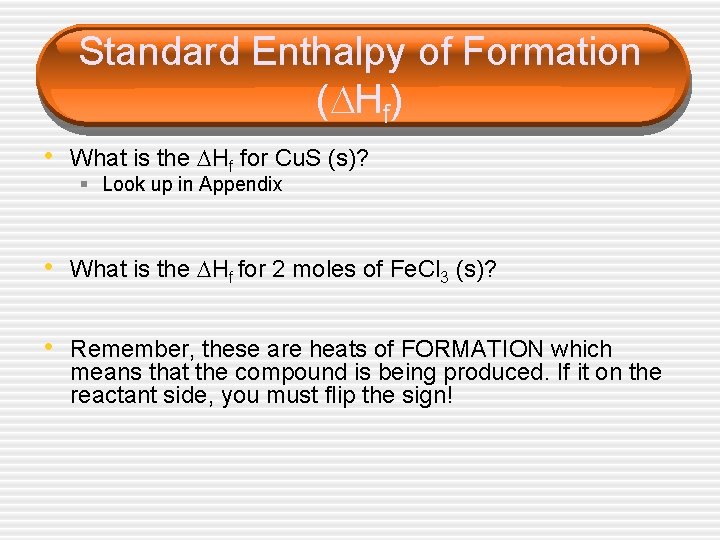
Standard Enthalpy of Formation ( Hf) • What is the Hf for Cu. S (s)? § Look up in Appendix • What is the Hf for 2 moles of Fe. Cl 3 (s)? • Remember, these are heats of FORMATION which means that the compound is being produced. If it on the reactant side, you must flip the sign!

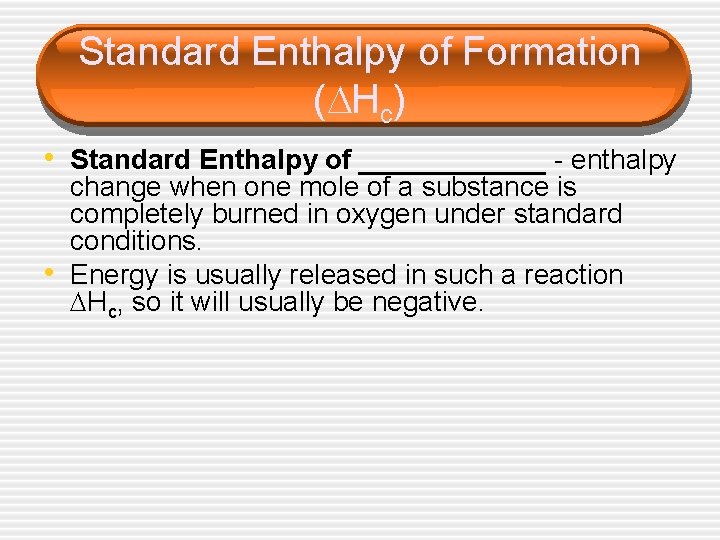
Standard Enthalpy of Formation ( Hc) • Standard Enthalpy of ______ - enthalpy • change when one mole of a substance is completely burned in oxygen under standard conditions. Energy is usually released in such a reaction Hc, so it will usually be negative.

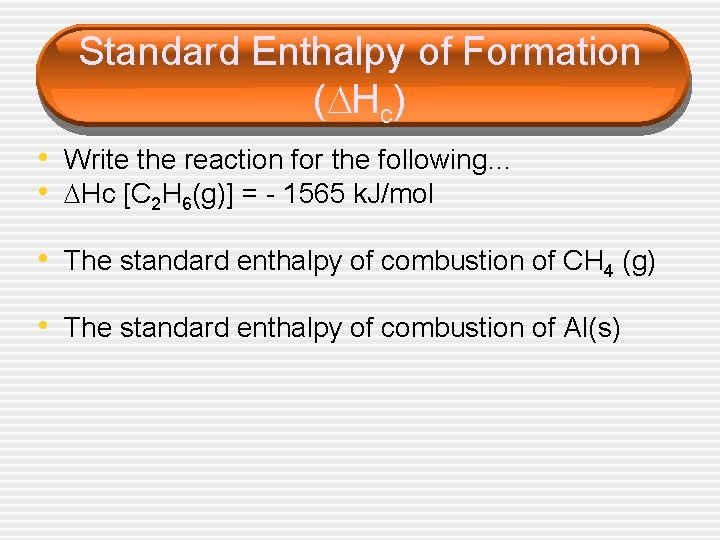
Standard Enthalpy of Formation ( Hc) • Write the reaction for the following… • Hc [C 2 H 6(g)] = - 1565 k. J/mol • The standard enthalpy of combustion of CH 4 (g) • The standard enthalpy of combustion of Al(s)

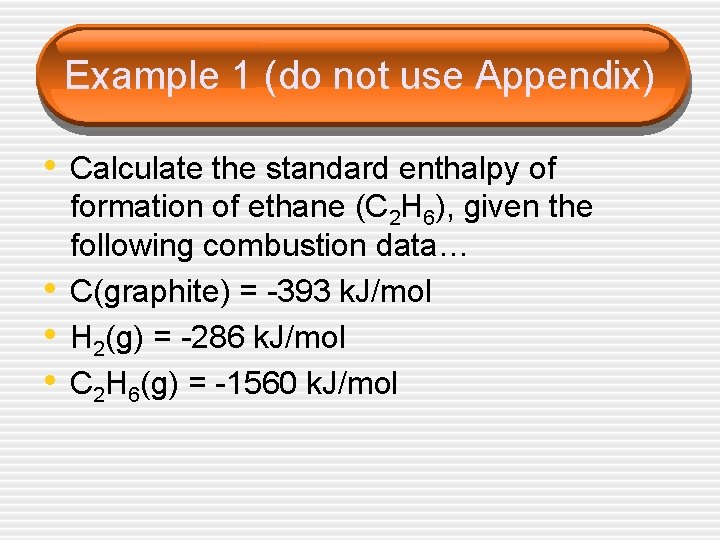
Example 1 (do not use Appendix) • Calculate the standard enthalpy of • • • formation of ethane (C 2 H 6), given the following combustion data… C(graphite) = -393 k. J/mol H 2(g) = -286 k. J/mol C 2 H 6(g) = -1560 k. J/mol

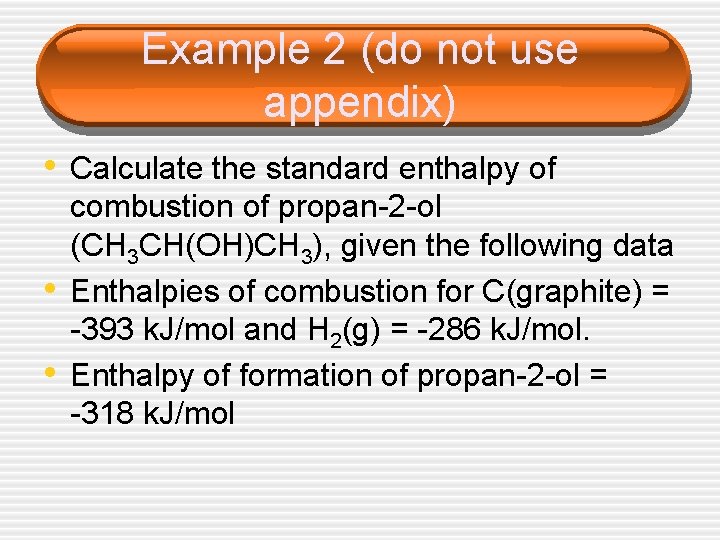
Example 2 (do not use appendix) • Calculate the standard enthalpy of • • combustion of propan-2 -ol (CH 3 CH(OH)CH 3), given the following data Enthalpies of combustion for C(graphite) = -393 k. J/mol and H 2(g) = -286 k. J/mol. Enthalpy of formation of propan-2 -ol = -318 k. J/mol

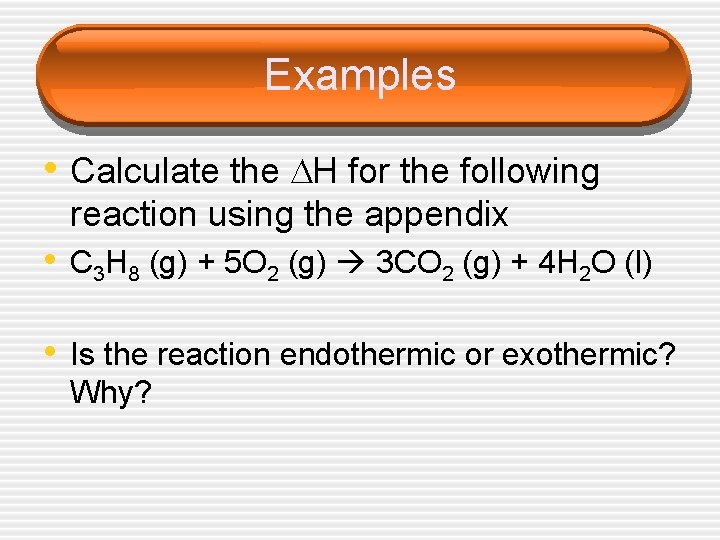
Examples • Calculate the H for the following reaction using the appendix • C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) • Is the reaction endothermic or exothermic? Why?

Examples • How much heat will be released from the combustion of 1. 80 g of C 6 H 6. Use the heat of formation data in the appendix.

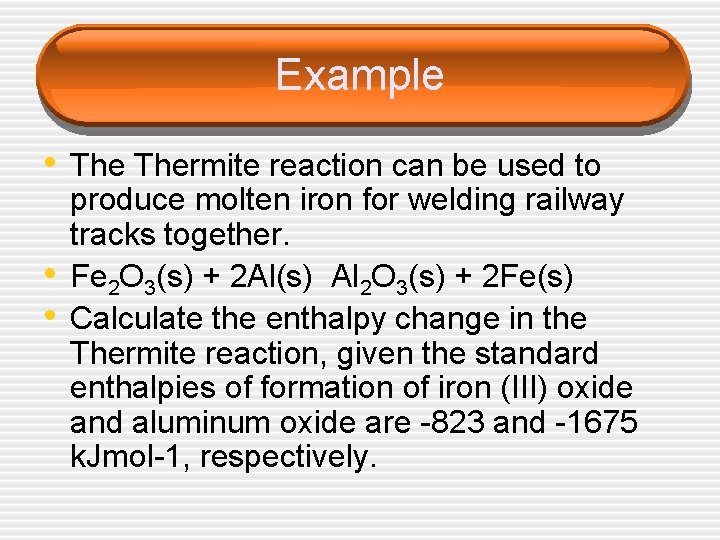
Example • Thermite reaction can be used to • • produce molten iron for welding railway tracks together. Fe 2 O 3(s) + 2 Al(s) Al 2 O 3(s) + 2 Fe(s) Calculate the enthalpy change in the Thermite reaction, given the standard enthalpies of formation of iron (III) oxide and aluminum oxide are -823 and -1675 k. Jmol-1, respectively.

Bond Enthalpies • The strength of the bond in a diatomic covalent molecule is given by the bond dissociation energy. • For example hydrogen, H 2 or H-H • H 2(g) 2 H(g) BDE= +436 k. J

Bond Enthalpies • In order to ______ a bond, energy must be put in (______ process) • When ______ a bond, energy is released (______ process).

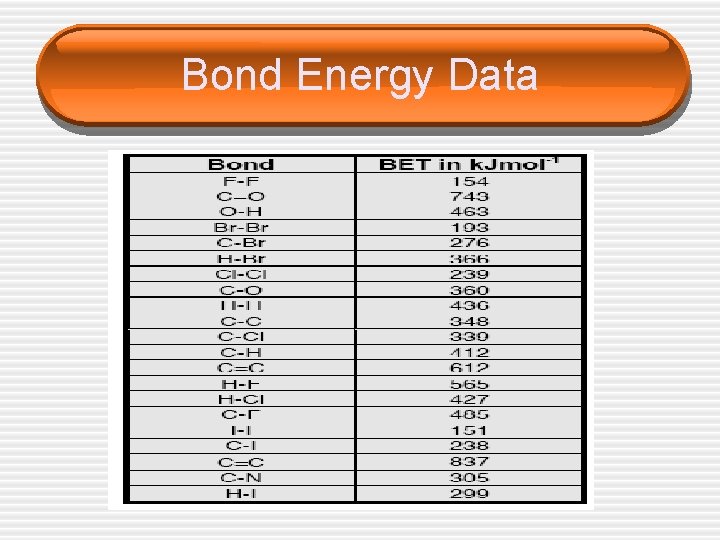
Bond Enthalpy Example 1 • Calculate the standard enthalpy of the • reaction below. CH 3 CH=CH 2 + H 2 CH 3 CH 2 CH 3

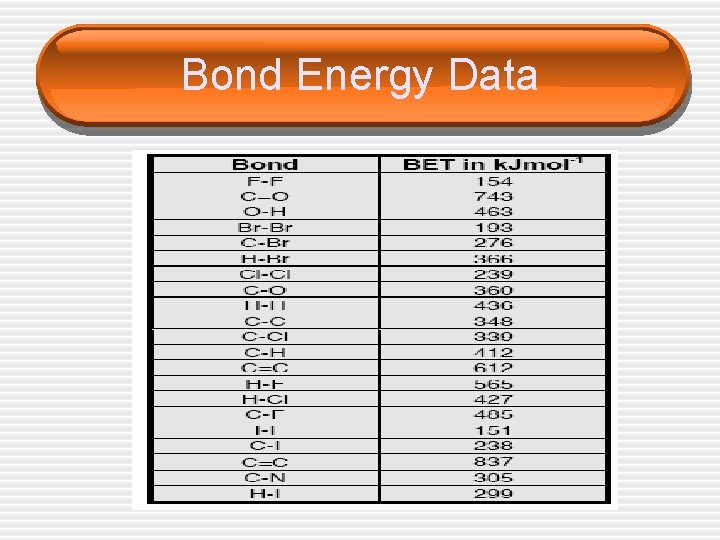
Bond Energy Data

Bond Enthalpy Example 2 • Calculate the enthalpy change for the • reaction below. CH 3 CH=CH 2 + Br 2 CH 2 Br. CH 3

Bond Energy Data

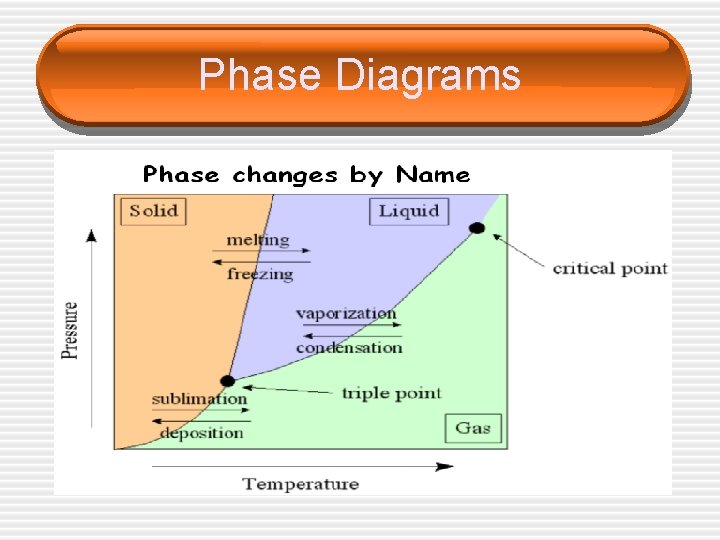
Phase Diagrams • An area on a phase diagram represents one ______ , a line represents the conditions under which two phases can exist in ______. • The ______ point describes the conditions under which all three phases can coexist. • The ______ point describes the maximum temperature that a liquid of the substance can exist. § Above this temperature the difference between the liquid and the gas disappear and the substance is referred to as a ______.

Phase Diagrams

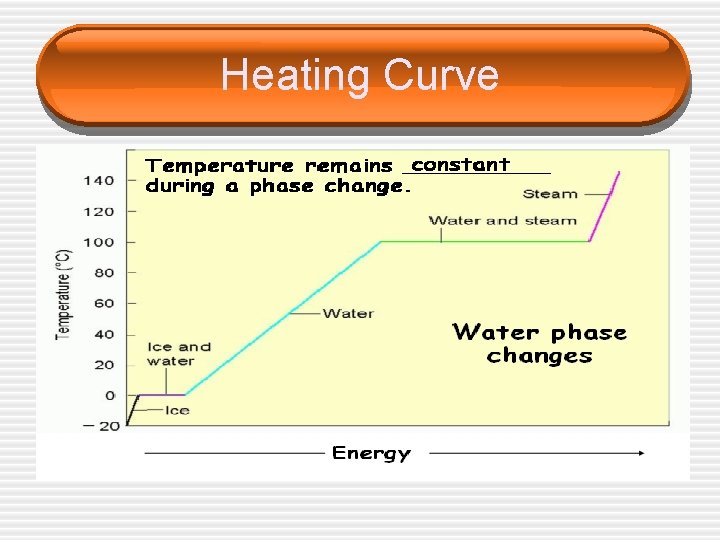
Heating Curve
 Hesss law
Hesss law Hesss law
Hesss law Hess law
Hess law Hesss
Hesss Kirchhoff's law of thermochemistry
Kirchhoff's law of thermochemistry Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law V=k/p
V=k/p Constant of avogadro's law
Constant of avogadro's law Formation initiale vs formation continue
Formation initiale vs formation continue What is standard molar enthalpy
What is standard molar enthalpy Gibbs free energy unit
Gibbs free energy unit Chapter 17 thermochemistry practice problems
Chapter 17 thermochemistry practice problems Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry is the study of: *
Thermochemistry is the study of: * Thermochemical equation
Thermochemical equation Thermochemistry
Thermochemistry Thermochemistry gaussian
Thermochemistry gaussian Thermochemical equation
Thermochemical equation Thermochemistry clipart
Thermochemistry clipart Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Thermochemistry is the study of
Thermochemistry is the study of Thermochemistry equations
Thermochemistry equations