THE MOLECULAR AND EMPIRICAL FORMULA GAME Can you
















- Slides: 16

THE MOLECULAR AND EMPIRICAL FORMULA GAME Can you find the molecular or empirical formula?

Question 1 ■ A compound has an empirical formula of Cl. CH 2 and its molar mass is 98. 96 g/mol. What is the molecular formula of this compound? – Cl 2 C 2 H 4

Question 2 ■ A compound consists of 75. 0% magnesium and 25. 0% oxygen. What is the empirical formula? – Mg 2 O

Question 3 ■ A compound has the following percent composition: 49. 48% carbon, 5. 19% hydrogen, 16. 48% oxygen, and 28. 85% nitrogen. What is the empirical formula? – C 4 H 5 ON 2

Question 4 ■ C 4 H 5 ON 2 has a molar mass of 194. 19 g/mol. What is its molecular formula – C 8 H 10 O 2 N 4

Question 5 ■ A compound is found to have 46. 67% nitrogen, 6. 70% hydrogen, 19. 98% carbon, and 26. 65% oxygen. What is its empirical formula? – N 2 H 4 CO

Question 6 ■ Find the empirical formula of a compound that contains 15. 8 g of Al, 28. 1 g of S, and 56. 1 g of oxygen. – Al 2 S 3 O 12 or Al 2(SO 4)3

Question 7 ■ Benzoic acid contains 68. 8% carbon, 4. 95% hydrogen, and 26. 25% oxygen. Find the empirical formula – C 7 H 6 O 2

Question 8 ■ Benzoic acid contains 68. 8% carbon, 4. 95% hydrogen, and 26. 25% oxygen. Find the empirical formula – C 7 H 6 O 2

Question 9 ■ A compound contains 50. 0% magnesium, 24. 0% carbon, 16. 0% oxygen, and 10. 0% hydrogen. What is the empirical formula? – Mg 2 C 2 OH 10

Question 10 ■ A compound contains 75. 46% carbon, 4. 43% hydrogen, and 20. 11% oxygen. It has a molar mass of 318. 31 g/mol. What is the empirical and molecular formula? – EF: C 10 H 7 O 2 – MF: C 20 H 14 O 4

Question 12 ■ Freons are gaseous compounds used in refrigeration. A particular Freon contains 9. 93% carbon, 58. 64% chlorine, and 31. 43% fluorine. What is the empirical formula – CCl 2 F 2

Question 12 ■ A compound with an empirical formula of CH is found to have a molar mass of 26. 04 g/mol. What is its molecular formula? – C 2 H 2

Question 13 ■ A compound consists of 40. 0% calcium, 12. 0% carbon, and 48. 0% oxygen. What is the empirical formula? – Ca. CO 3

Question 14 ■ The empirical formula for a hydrocarbon is CH 2. Its actual molar mass is 294 g/mol. What is its molecular formula? – C 21 H 42

Question 15 ■ A compound consists of 85. 0% silver and 15. 0% fluorine. What is the empirical formula? – Ag. F
 Molecular formula from empirical formula
Molecular formula from empirical formula What are the empirical formulas
What are the empirical formulas Emphrical formula
Emphrical formula Chapter 7 review chemical formulas and chemical compounds
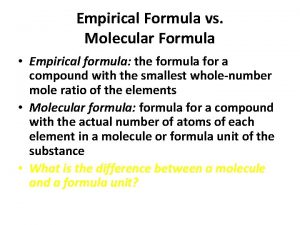
Chapter 7 review chemical formulas and chemical compounds Empirical formula vs
Empirical formula vs Empirical formula rhyme
Empirical formula rhyme Empirical formula vs molecular formula
Empirical formula vs molecular formula Calculating percentage composition by mass
Calculating percentage composition by mass Empirical formula
Empirical formula Empirical formula rhyme
Empirical formula rhyme Empirical formula to percent composition
Empirical formula to percent composition Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet Molar mass quiz
Molar mass quiz Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Empirical formula poem
Empirical formula poem If you think you can you can poem
If you think you can you can poem