The Johns Hopkins University School of Medicine Office


























- Slides: 45

The Johns Hopkins University School of Medicine Office of Research Administration Subgrants & Subcontracts Training Nov 2014 1

Our Work is Divided into Two Categories… Drafting, execution, and termination of outgoing subgrants and subcontracts Purchase order creation, management, and termination 2

�A When is an Outgoing Subaward Necessary? subgrant or subcontract is necessary when: ◦ The site will perform work which satisfies a direct objective or deliverable of the Prime Award ◦ The site will be responsible for programmatic decisions or design related to the project/study under the Prime Award ◦ The site will be held accountable to the compliance regulations of the Prime Award (COI, IP, etc) or may seek to publish/co author the study results ◦ The site will use the Prime Award funds to carry out its own programmatic work ◦ The site provides cost sharing or has key personnel mentioned in the NGA 3

Other Types of Agreements… (Not Drafted by Subawards Team) � Service/Vendor Agreement (drafted by Purchasing, PO set up by dept): ◦ If the site is providing standard services (those in the normal operations and markets to a variety of customers) ◦ If the site has not/will not provide significant contributions to the design/conduct of the project ◦ If the site is not responsible for the project results ◦ If the site performs work that involves routine or repeated activities ◦ If the site will not seek to publish or co-author based on the academic standards such as the ICJME standards � � Storage (drug, supplies, etc. ) Website design & management for a clinical trial. KKI Kirby Center Agreements Draft Examples: http: //ssc. jhmi. edu/supplychain/forms/index. html Data Use Agreement � Complex Material Transfer Agreement � Collaboration Agreement � 4

Types of Prime Sponsor Funding… � Subgrants under Federal grants � Subcontracts under Federal and state contracts � Subgrants under awards from foundations � Subcontracts under commercial contracts � Funding from multiple sources ◦ Please try to make us aware of all the sources of funding (commercial and non-commercial) for the subaward � Internal JHU awards 5

Internal JHU Funding/Other… � NTAP – Neurofibromatosis Therapeutic Acceleration Program � ABTC – Adult Brain Tumor Consortium � TBCRC – Translational Breast Cancer Consortium � PACKARD – The Robert Packard Center for ALS Research � CRN – Clinical Research Network (AAHSRI, GBMC, INOVA, PRMC, RHMC) 6

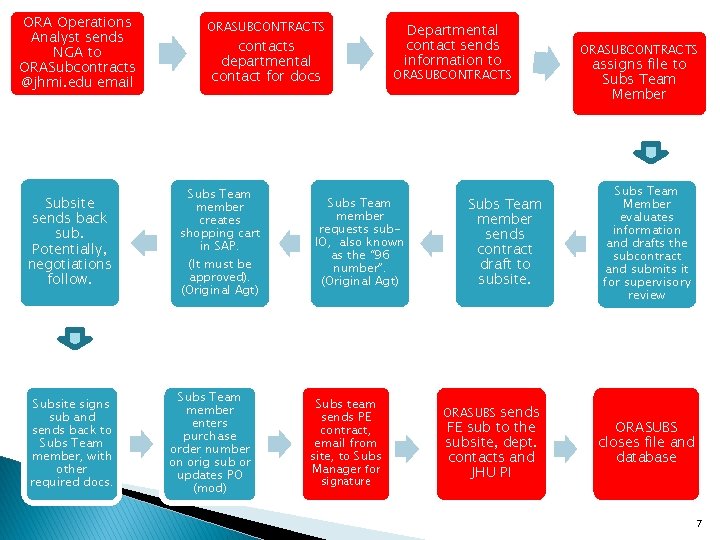
ORA Operations Analyst sends NGA to ORASubcontracts @jhmi. edu email Subsite sends back sub. Potentially, negotiations follow. Subsite signs sub and sends back to Subs Team member, with other required docs. ORASUBCONTRACTS contacts departmental contact for docs Subs Team member creates shopping cart in SAP. (It must be approved). (Original Agt) Subs Team member enters purchase order number on orig sub or updates PO (mod) Departmental contact sends information to ORASUBCONTRACTS Subs Team member requests sub. IO, also known as the “ 96 number”. (Original Agt) Subs team sends PE contract, email from site, to Subs Manager for signature Subs Team member sends contract draft to subsite. ORASUBS sends FE sub to the subsite, dept. contacts and JHU PI ORASUBCONTRACTS assigns file to Subs Team Member evaluates information and drafts the subcontract and submits it for supervisory review ORASUBS closes file and database 7

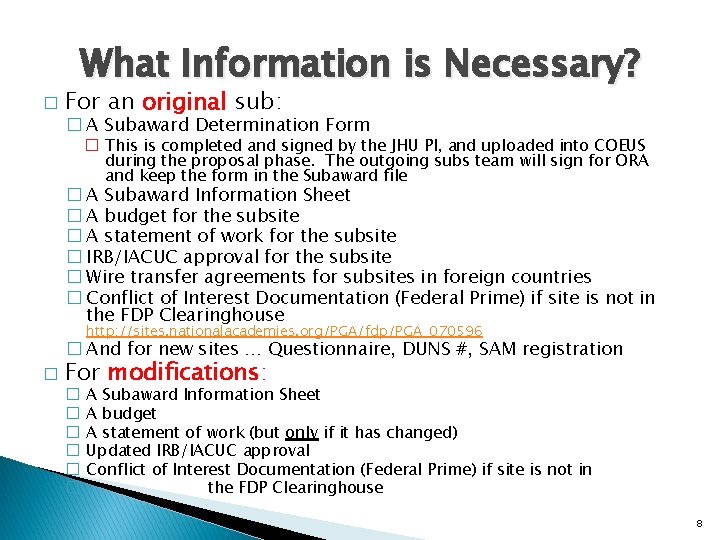
What Information is Necessary? � For an original sub: � A Subaward Determination Form � This is completed and signed by the JHU PI, and uploaded into COEUS during the proposal phase. The outgoing subs team will sign for ORA and keep the form in the Subaward file � A Subaward Information Sheet � A budget for the subsite � A statement of work for the subsite � IRB/IACUC approval for the subsite � Wire transfer agreements for subsites in foreign countries � Conflict of Interest Documentation (Federal Prime) if site is not in the FDP Clearinghouse http: //sites. nationalacademies. org/PGA/fdp/PGA_070596 � And for new sites … Questionnaire, DUNS #, SAM registration � For modifications: � � � A Subaward Information Sheet A budget A statement of work (but only if it has changed) Updated IRB/IACUC approval Conflict of Interest Documentation (Federal Prime) if site is not in the FDP Clearinghouse 8

Who Does the Department Submit Outgoing Subaward Paperwork to? � � � ORASUBCONTRACTS@jhmi. edu for processing and assignment to a Subawards Team Member Subawards Specialists draft subs under Federal Grants When the prime award is a federal contract, a commercial contract, or an award from a foundation, it will be handled by: ◦ Melody: �Original subcontracts under federal and all commercial contracts �CRN SSA’s, NTAP, TBCRC, internal accounts ◦ Michelle: �Subs under foundation awards �Modifications of subcontracts under federal contracts �ABTC 9

Subcontract Information Sheet… � � � � � JHU contact information � The “Financial Contact” is the person who processes invoices and goods receipts � The “Study Contact” is the person who can answer scientific questions about the protocol. Most recent subsite contact information � In most cases, the contact should be in the Research Administration office at the subsite. � Verify that it is current each year an Information Sheet is submitted. SAP grant #, COEUS IPN #, Prime award/contract IO #, PI’s cost center # Sponsor grant/contract # (JHU is only the “sponsor” under 800 act's) Deliverables and due dates/time-lines for new/revised SOW’s Whether animal and/or human subjects research is going to be conducted at the subsite. Budget Period for the sub & amount to pay the subsite (PO amount) Information related to the Study for Clinical Trials Information related to Transfer of drug, equipment or materials. 10

Budgets… � Budgets include: for subs under Federal grants should �Names of personnel �Breakdown of costs for equipment, supplies, and “other expenses” �Explanation of travel costs � In all budgets, if the site is going to be paid for each patient it accrues, or for each sample it obtains, etc. , the budget must indicate the number of patients/samples and the amount that will be paid for each one. � Consider whether the costs are allowed under the terms of the award/contract. 11

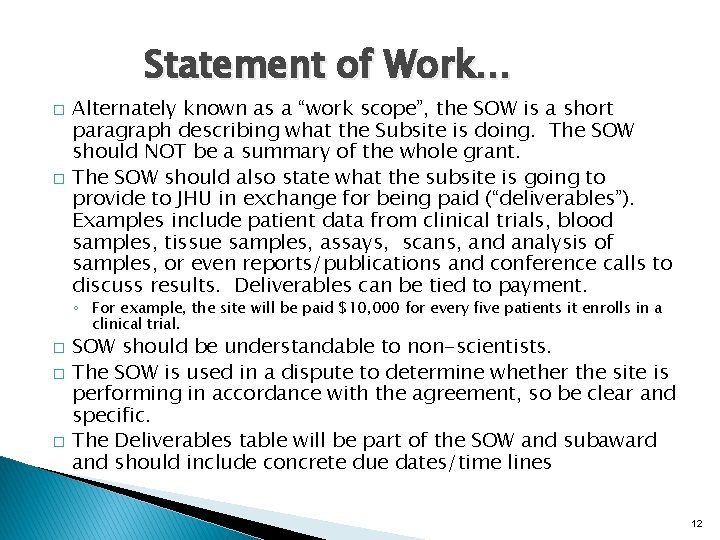
Statement of Work… � � Alternately known as a “work scope”, the SOW is a short paragraph describing what the Subsite is doing. The SOW should NOT be a summary of the whole grant. The SOW should also state what the subsite is going to provide to JHU in exchange for being paid (“deliverables”). Examples include patient data from clinical trials, blood samples, tissue samples, assays, scans, and analysis of samples, or even reports/publications and conference calls to discuss results. Deliverables can be tied to payment. ◦ For example, the site will be paid $10, 000 for every five patients it enrolls in a clinical trial. � � � SOW should be understandable to non-scientists. The SOW is used in a dispute to determine whether the site is performing in accordance with the agreement, so be clear and specific. The Deliverables table will be part of the SOW and subaward and should include concrete due dates/time lines 12

IRB and IACUC approvals � It is up to the Subsite’s IRB/IACUC to determine whether human subjects and/or vertebrate animals research is being conducted. � ORA does NOT need the Subsite IRB approval to send the Agreement, but we must have it to fully-execute the Agreement. � KKI and the CRN site Study Team Members should be included as part of the JHU IRB approval prior to submission of paperwork to ORASUBS 13

Wire transfer agreements �Contains bank account and payment information for the Subsite. �Is attached to the Subaward as an exhibit �Must be copied onto Subsite letterhead �New foreign vendors also require a W 8 if the site is using a domestic bank � NOTE: new domestic vendors may also require a W 9 so that a vendor number can be requested for the PO 14

Special Considerations for High Risk Subsites Under Federal Funds… � Under Federal grants and contracts, all Subrecipients should be registered in the System for Award Management (“SAM”), and must have a DUNS number ◦ SAM website: https: //sam. gov ◦ DUNS number requests: http: //fedgov/dnd. com/webform � High Risk Subrecipient Monitoring: ◦ ORASUBS will identify sites that need to complete a Subrecipient Questionnaire ◦ The Dept will send the Welcome Packet and Questionnaire to the identified sites and return it to ORASUBS ◦ ORA rates the sites and notifies the dept and Internal Audits who confirms the rating and performs subsite audits ◦ High Risk Subrecipients must submit invoices on template included as exhibit to the cost reimbursement Sub Agreement. ◦ The JHU dept must complete additional invoice audits per the new risk management regulations which took effect on 7/1/14. 15

� As Conflict of Interest Under Federal Funds… of 8/24/12: Subsites are required to submit COI information at the time of JHU application to the JHU Grants Associates if they are not registered in the FDP Clearinghouse. � 2 Options: ◦ Complete, and certify best ability at time of JHU application ◦ Opt to complete certification at time of Subagreement award � Subs team will choose appropriate COI language for the Agreement � If SCF was not completed at time of JHU application and the site is not registered in the FDP Clearinghouse, Subs team will request that the dept get an SFI from the Subsite prior to submission of Subaward documents to ORASUBCONTRACTS. 16

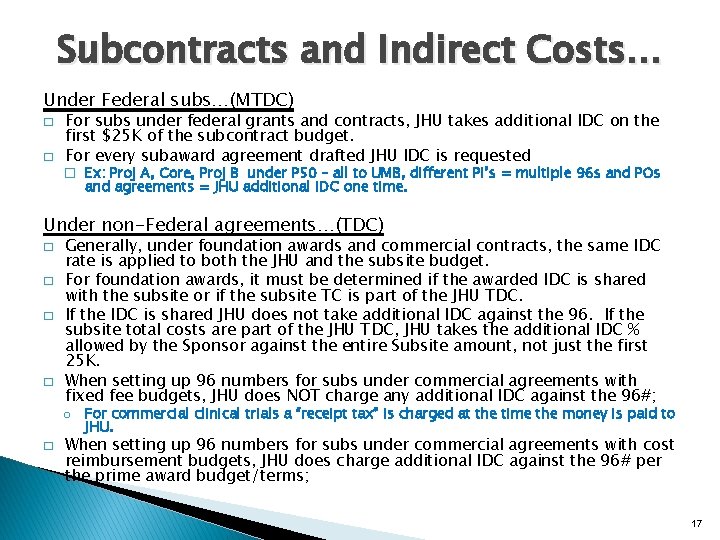
Subcontracts and Indirect Costs… Under Federal subs…(MTDC) � � For subs under federal grants and contracts, JHU takes additional IDC on the first $25 K of the subcontract budget. For every subaward agreement drafted JHU IDC is requested � Ex: Proj A, Core, Proj B under P 50 – all to UMB, different PI’s = multiple 96 s and POs and agreements = JHU additional IDC one time. Under non-Federal agreements…(TDC) � � Generally, under foundation awards and commercial contracts, the same IDC rate is applied to both the JHU and the subsite budget. For foundation awards, it must be determined if the awarded IDC is shared with the subsite or if the subsite TC is part of the JHU TDC. If the IDC is shared JHU does not take additional IDC against the 96. If the subsite total costs are part of the JHU TDC, JHU takes the additional IDC % allowed by the Sponsor against the entire Subsite amount, not just the first 25 K. When setting up 96 numbers for subs under commercial agreements with fixed fee budgets, JHU does NOT charge any additional IDC against the 96#; o For commercial clinical trials a “receipt tax” is charged at the time the money is paid to JHU. � When setting up 96 numbers for subs under commercial agreements with cost reimbursement budgets, JHU does charge additional IDC against the 96# per the prime award budget/terms; 17

The 96 Number… The 96 number simply refers to the SAP account number for the sub. � A 96 number is a “child” of a “parent” Internal Order number (IO), which will always start with “ 900”. � The 96 # will be set up with the sub amount, plus whatever IDC JHU is entitled to take. � o Except in the case of commercial clinical trials and most foundation awards. Generally speaking, the IDC rate for the 96 # must match the IDC rate for the IO # it is based upon, although there may be some (limited) exceptions. � After the first year of the Subaward, the departments are responsible for distributing money to the 96 #. � 18

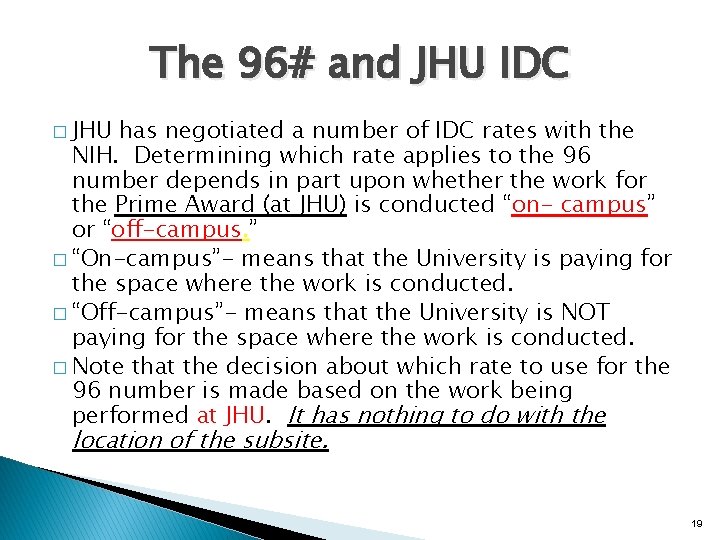
The 96# and JHU IDC � JHU has negotiated a number of IDC rates with the NIH. Determining which rate applies to the 96 number depends in part upon whether the work for the Prime Award (at JHU) is conducted “on- campus” or “off-campus. ” � “On-campus”- means that the University is paying for the space where the work is conducted. � “Off-campus”- means that the University is NOT paying for the space where the work is conducted. � Note that the decision about which rate to use for the 96 number is made based on the work being performed at JHU. It has nothing to do with the location of the subsite. 19

Carry Over of Funding… ØORA’s default position on carry over: subs follow the terms of the prime grant or contract ØHowever, even in cases where the prime grant/contract allows for automatic carryover, the department and PI may request ORA to set up subs so that the subsite must obtain authorization to carry over any funding. ØIn order for funding to be carried over under Federal funding, the balance should be reported on the Financial Status Report as an “unliquidated obligation. ” ØIf the prime award is from a foundation, the carryover process should comply with award guidelines about how much money can be carried over, etc. 20

Carryover Modifications… � Carryover modifications are always necessary when the prime grant or contract requires JHU to obtain authorization for carrying over prime grant/contract funding. ◦ Almost always necessary for P and U awards �A carryover modification authorizes the subsite to carryover funding from one subgrant/contract budget period to another. � Carryover modifications must specify the amount of funding being carried over, but do not need to include a whole new budget. 21

The Federal Funding Accountability and Transparency Act (FFATA)… � The Federal Funding Accountability and Transparency Act (“FFATA”) was a 2006 federal law intended to hold the government accountable for its spending by allowing individuals to track information on federal grants and contracts on a searchable website (www. USASpending. gov) � Under FFATA, recipients of federal grants and contracts must report Subaward and Subcontract spending. �The SOM Subawards Coordinator will handle the reporting �Subsites will be asked to provide certain information to facilitate the reporting process. 22

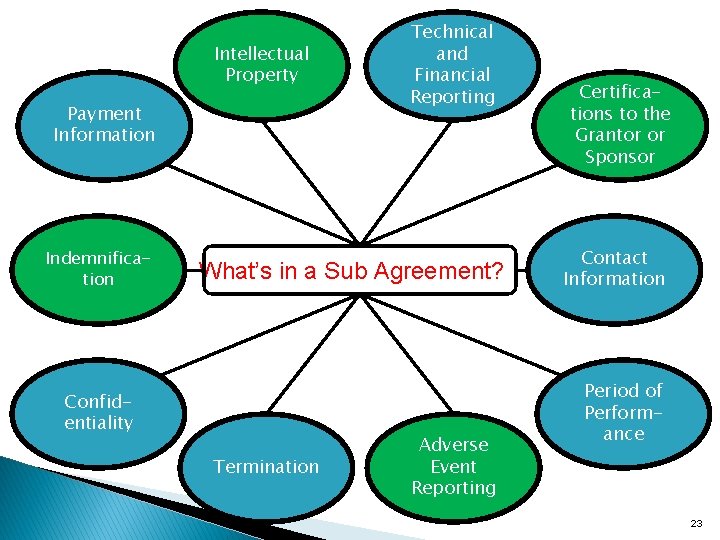
Intellectual Property Payment Information Indemnification Technical and Financial Reporting What’s in a Sub Agreement? ? Confidentiality Termination Adverse Event Reporting Certifications to the Grantor or Sponsor Contact Information Period of Performance 23

Managing Purchase Orders What the Subs group does in relation to purchase orders: §Creation: §P. O. is created when the contract is created. §Modification: §Time and/or money added (or removed) when sub is modified. §Close-out: §P. O. is closed when project period of grant/contract ends, or sub is terminated early. If money is left on 96#, the close out process will unencumber it. **Do NOT have Purchasing or SAP help do these things!** 24

Closing out PO’s § ORA closes the Purchase Order once the final invoice is approved, submitted to AP, confirmed and paid. If the period of performance for the subaward has expired, the department sends the ORA Subawards Team (ORASUBCONTRACTS@jhmi. edu) and email with the PO number indicating to close it. PLEASE DO NOT EMAIL PURCHASING/AP/SPSS/SAP/PO MAINTENANCE FIRST! § The Subawards Team does not move money off of or unencumber money from the 96. If this does not automatically happen when the PO closes, please reach out to Purchasing@jhu. edu or SAPHelp@jhu. edu for more help. § If there were expenses on a P. O. before the January 2007 conversion to SAP, they will show up on the 642400 general ledger number, which links to the IO number, NOT to the 96 number. Thus, if we are closing a P. O. , and there is money on 642400, the Real Time Grant Report will still show that there is an encumbrance on the IO number. ORA cannot fix this problem. For help, contact the SAP help line and/or Purchasing. 25

Closing out PO’s/Subawards � � There are several situations where a Subaward might need to be closed out before the period of performance expires. If the period of performance has not expired AND a final invoice has been paid: ◦ The department should send an infosheet to ORASUBCONTRACTS ◦ On the infosheet the period of performance should start on the date of their last agreement and end on the final invoiceable date ◦ On the infosheet the amount should be the total amount paid for that period of performance ◦ ORA will send the site a termination modification AND close out the PO/SC once the mod is fully executed The period of performance has expired AND a final invoice has not been sent: ◦ The department should send an infosheet to ORASUBCONTRACTS ◦ On the infosheet the period of performance should start on the date of their last agreement and end on the last date the sub PI completed work for JHU ◦ ORA will send the site a termination modification ◦ The department should email ORASUBCONTRACTS when the final invoice is paid ◦ ORA will close out the PO/SC at that time 26

Closing out PO’s/Subawards � If the subsite PI is moving to another subsite and will still conduct work for JHU under the project ◦ The department should request paperwork from ORASUBCONTRACTS for a new subsite ◦ The department should complete and return the paperwork to ORASUBCONTRACTS ◦ ORA will draft and submit the new sub AND create new 96/PO � If the subsite is appointing another sub-PI to do the work at their Institution ◦ The department should send an infosheet to ORASUBCONTRACTS with the new PI’s information on it and a new budget for that PI for the remaining portion of the current grant year ◦ ORA will modify the current agreement with the subsite ◦ The existing 96/PO will continue to be used 27

What the Departments do in relation to purchase orders: • Process and pay invoices • Complete goods receipts • Ensure consistency with Real Time Grant Report and BW reports 28

Invoice Process Per the terms of our contracts, subsites should send invoices to the department financial contact on the sub information sheet. � If ORA receives an invoice erroneously, it will be forwarded to the Financial Contact for the sub. � Confirm with the PI that the work has been performed and that any necessary reports have been submitted. PI should be aware of payments. � Certification for Payment should be completed and signed by PI � Departmental contact sends invoice and Certification to Accounts Payable via e-mail. Make sure to keep a copy. The e-mail should indicate if the invoice should be split between lines on the purchase order and how much to put on each line. � For Fixed Fee/Per Patient budgets, dept should send an email to ORASUBS asking for money to be added to the PO BEFORE submission of the invoice to AP. � 29

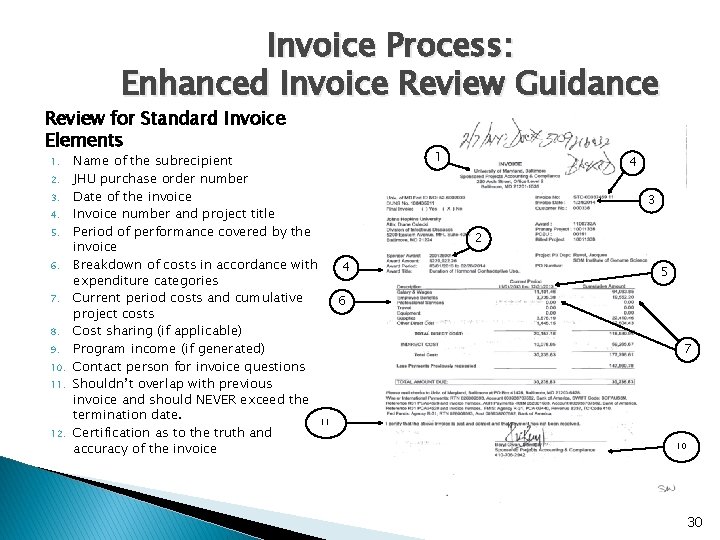
Invoice Process: Enhanced Invoice Review Guidance Review for Standard Invoice Elements 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Name of the subrecipient JHU purchase order number Date of the invoice Invoice number and project title Period of performance covered by the invoice Breakdown of costs in accordance with 4 expenditure categories Current period costs and cumulative 6 project costs Cost sharing (if applicable) Program income (if generated) Contact person for invoice questions Shouldn’t overlap with previous invoice and should NEVER exceed the termination date. 11 Certification as to the truth and accuracy of the invoice 1 4 3 2 5 7 10 30

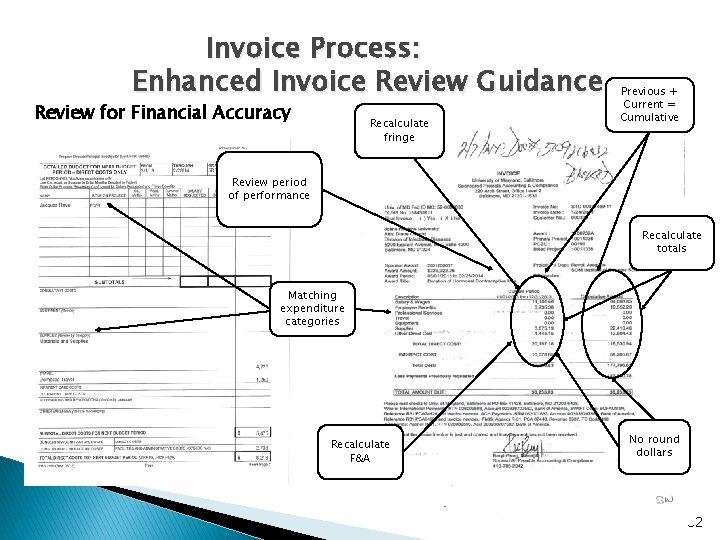
Invoice Review: Financial Accuracy Ø Review for standard invoice elements as required by current policy (e. g. , cumulative costs, certification etc. ) Ø Expense in line with budget category amounts Ø Amounts aren’t round dollars solely based on a percentage of budget Ø Previous expense + Current expense = Cumulative expense for each budget category Ø Column totals equal the amount of individual column amounts in total Ø Fringe cost are in line with salary expense for the agreed upon fringe rate Ø Facilities and Administrative (F&A) costs are in line with applicable costs for the agreed upon F&A rate Ø Period of performance invoiced is not in advance of the current calendar date unless immediate cash needs are being met. If so, cash balances and interest must be tracked and reconciled before additional payments can be made. Ø Be sure that cumulative amount invoiced is less than or equal to total amount of the subaward/subcontract. 31

Invoice Process: Enhanced Invoice Review Guidance Review for Financial Accuracy Recalculate fringe Previous + Current = Cumulative Review period of performance Recalculate totals Matching expenditure categories Recalculate F&A No round dollars 32

Invoice Process: Certification for Payment form � � � Certification for Payment and Performance must be completed for all invoices Confirm with the PI that the work has been performed and that any necessary reports have been submitted. PI should be aware of payments. Approval by both Financial Analyst and Principal Investigator is required Form must be included with invoice in order to process payment in SAP Form is emailed to JHU PI with every fully executed agreement and is located on JHU Finance website. https: //apps. finance. jhu. edu/policy_procedures/policy/sub/cert_pymt_perf. docx 33

Invoice Process: Enhanced Invoice Review Guidance 34

Invoice Process: High Risk Monitoring � � � High-risk subrecipients will be required to submit general ledger summary and detailed financial reports along with all invoices Departmental personnel will be responsible for completing a summary comparison of the invoice to the financial reports Every six months, departmental personnel will complete a more detailed review of a subrecipient financial report supporting one invoice, including obtaining supporting documentation for a selection of charges The Certification for Payment and Performance form has been revised to evidence these reviews ◦ A summary review is required to be performed and must be documented on the Certification for Payment and Performance ◦ Date of last detailed review must also be documented Problems identified with billings must be resolved and the resolution documented 35

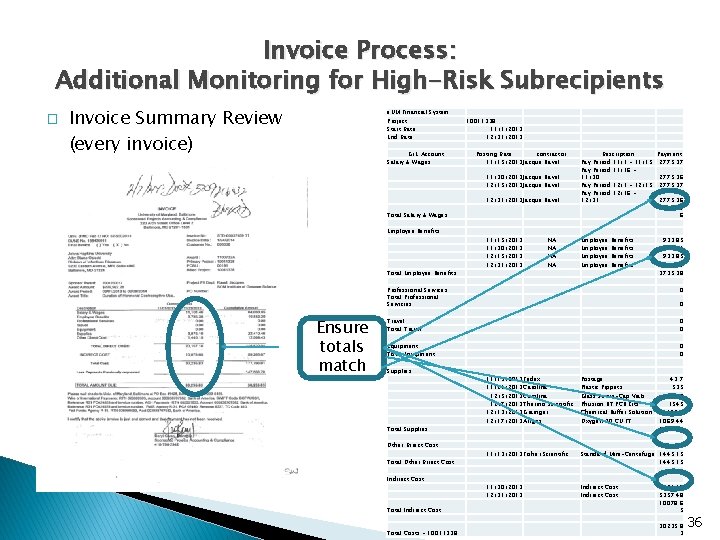
Invoice Process: Additional Monitoring for High-Risk Subrecipients � Invoice Summary Review (every invoice) e. UM Financial System Project Start Date End Date G/L Account Salary & Wages 10011338 11/1/2013 12/31/2013 Posting Date contractor 11/15/2013 Jacque Ravel 11/30/2013 Jacque Ravel 12/15/2013 Jacque Ravel 12/31/2013 Jacque Ravel Total Salary & Wages Employee Benefits Total Employee Benefits Ensure totals match 11/15/2013 11/30/2013 12/15/2013 12/31/2013 NA NA Description Payment Pay Period 11/1 - 11/15 2775. 37 Pay Period 11/16 11/30 2775. 36 Pay Period 12/1 - 12/15 2775. 37 Pay Period 12/16 12/31 2775. 36 11101. 4 6 Employee Benefits 933. 85 933. 84 3735. 38 Professional Services Total Professional Servicies 0 Travel Total Travel 0 0 Equipment Total Equipment 0 0 Supplies Total Supplies Other Direct Cost Total Other Direct Cost Indirect Cost 0 11/13/2013 Fedex 11/21/2013 Carolina 12/5/2013 Carolina 12/7/2013 Thermo Scientific 12/13/2013 Grainger 12/17/2013 Airgas Postage Plastic Pippets Glass Screw-Cap Vials Phusion RT PCR Kits Chemical Buffer Solution Oxygen 20 CU FT 43. 7 535 526. 25 1545 155. 8 1069. 44 3875. 19 11/13/2013 Fisher Scientific Standard Mini-Centrifuge 1445. 15 11/30/2013 12/31/2013 Indirect Cost Total Indirect Cost 4721. 17 5357. 48 10078. 6 5 Total Costs - 10011338 30235. 8 3 36

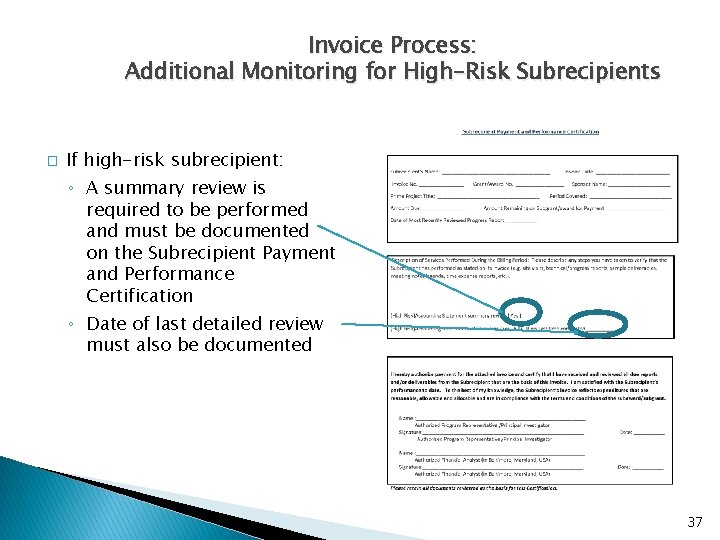
Invoice Process: Additional Monitoring for High-Risk Subrecipients � If high-risk subrecipient: ◦ A summary review is required to be performed and must be documented on the Subrecipient Payment and Performance Certification ◦ Date of last detailed review must also be documented 37

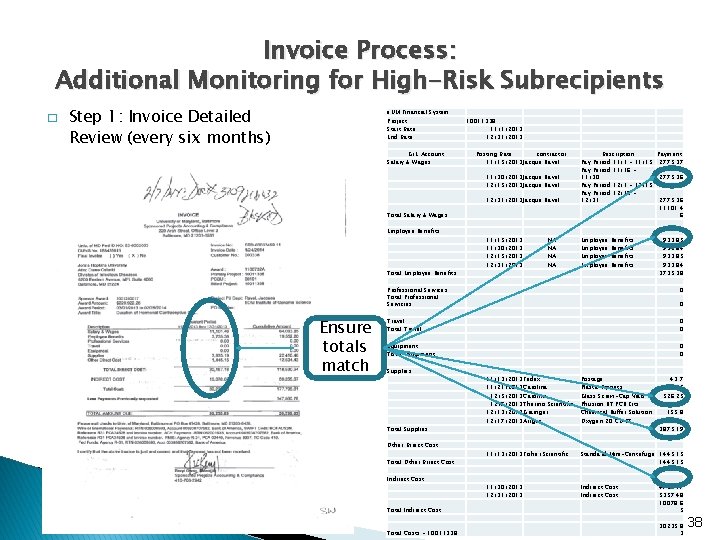
Invoice Process: Additional Monitoring for High-Risk Subrecipients � Step 1: Invoice Detailed Review (every six months) e. UM Financial System Project Start Date End Date G/L Account Salary & Wages 10011338 11/1/2013 12/31/2013 Posting Date contractor 11/15/2013 Jacque Ravel 11/30/2013 Jacque Ravel 12/15/2013 Jacque Ravel 12/31/2013 Jacque Ravel Total Salary & Wages Employee Benefits Total Employee Benefits Ensure totals match 11/15/2013 11/30/2013 12/15/2013 12/31/2013 NA NA Description Payment Pay Period 11/1 - 11/15 2775. 37 Pay Period 11/16 11/30 2775. 36 Pay Period 12/1 - 12/15 2775. 37 Pay Period 12/16 12/31 2775. 36 11101. 4 6 Employee Benefits 933. 85 933. 84 3735. 38 Professional Services Total Professional Servicies 0 Travel Total Travel 0 0 Equipment Total Equipment 0 0 Supplies Total Supplies Other Direct Cost Total Other Direct Cost Indirect Cost 0 11/13/2013 Fedex 11/21/2013 Carolina 12/5/2013 Carolina 12/7/2013 Thermo Scientific 12/13/2013 Grainger 12/17/2013 Airgas Postage Plastic Pippets Glass Screw-Cap Vials Phusion RT PCR Kits Chemical Buffer Solution Oxygen 20 CU FT 43. 7 535 526. 25 1545 155. 8 1069. 44 3875. 19 11/13/2013 Fisher Scientific Standard Mini-Centrifuge 1445. 15 11/30/2013 12/31/2013 Indirect Cost Total Indirect Cost 4721. 17 5357. 48 10078. 6 5 Total Costs - 10011338 30235. 8 3 38

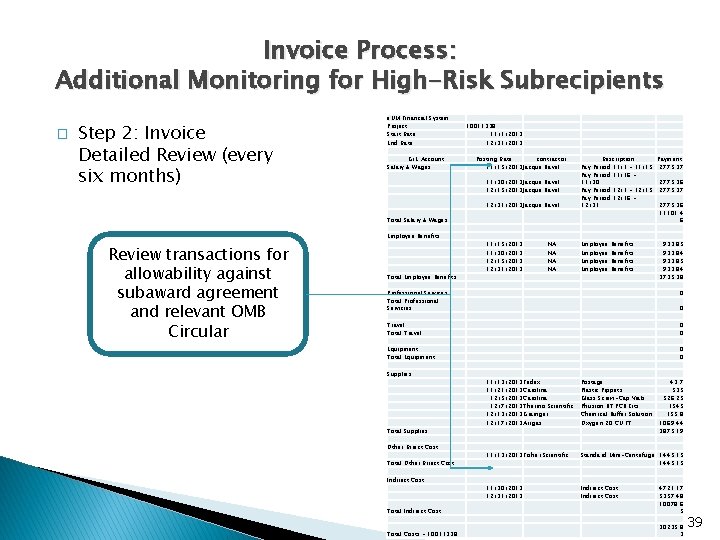
Invoice Process: Additional Monitoring for High-Risk Subrecipients � Step 2: Invoice Detailed Review (every six months) e. UM Financial System Project Start Date End Date G/L Account Salary & Wages 10011338 11/1/2013 12/31/2013 Posting Date contractor 11/15/2013 Jacque Ravel 11/30/2013 Jacque Ravel 12/15/2013 Jacque Ravel 12/31/2013 Jacque Ravel Total Salary & Wages Employee Benefits Review transactions for allowability against subaward agreement and relevant OMB Circular Total Employee Benefits 11/15/2013 11/30/2013 12/15/2013 12/31/2013 NA NA Description Payment Pay Period 11/1 - 11/15 2775. 37 Pay Period 11/16 11/30 2775. 36 Pay Period 12/1 - 12/15 2775. 37 Pay Period 12/16 12/31 2775. 36 11101. 4 6 Employee Benefits 933. 85 933. 84 3735. 38 Professional Services Total Professional Servicies 0 Travel Total Travel 0 0 Equipment Total Equipment 0 0 Supplies Total Supplies Other Direct Cost Total Other Direct Cost Indirect Cost 0 11/13/2013 Fedex 11/21/2013 Carolina 12/5/2013 Carolina 12/7/2013 Thermo Scientific 12/13/2013 Grainger 12/17/2013 Airgas Postage Plastic Pippets Glass Screw-Cap Vials Phusion RT PCR Kits Chemical Buffer Solution Oxygen 20 CU FT 43. 7 535 526. 25 1545 155. 8 1069. 44 3875. 19 11/13/2013 Fisher Scientific Standard Mini-Centrifuge 1445. 15 11/30/2013 12/31/2013 Indirect Cost Total Indirect Cost 4721. 17 5357. 48 10078. 6 5 Total Costs - 10011338 30235. 8 3 39

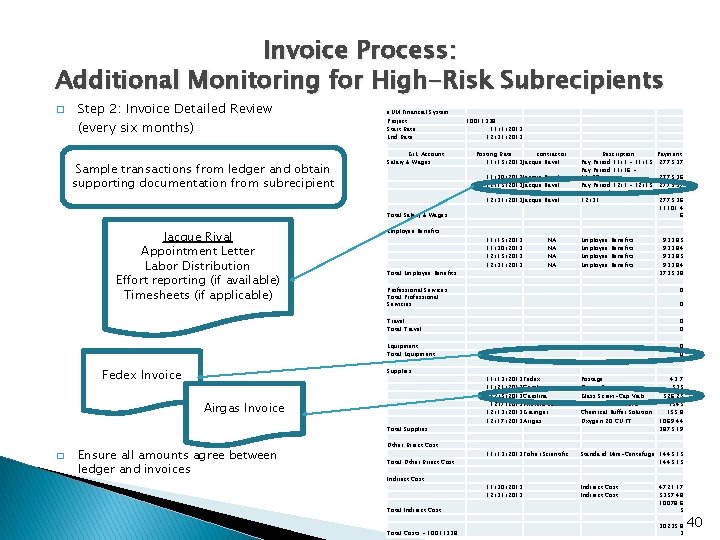
Invoice Process: Additional Monitoring for High-Risk Subrecipients � Step 2: Invoice Detailed Review (every six months) Sample transactions from ledger and obtain supporting documentation from subrecipient e. UM Financial System Project Start Date End Date G/L Account Salary & Wages 10011338 11/1/2013 12/31/2013 Posting Date contractor 11/15/2013 Jacque Ravel 11/30/2013 Jacque Ravel 12/15/2013 Jacque Ravel 12/31/2013 Jacque Ravel Total Salary & Wages Jacque Rival Appointment Letter Labor Distribution Effort reporting (if available) Timesheets (if applicable) Employee Benefits Total Employee Benefits Benefits 933. 85 933. 84 3735. 38 Travel Total Travel 0 0 Equipment Total Equipment 0 0 Total Supplies Ensure all amounts agree between ledger and invoices Employee 0 Airgas Invoice � NA NA Professional Services Total Professional Servicies Supplies Fedex Invoice 11/15/2013 11/30/2013 12/15/2013 12/31/2013 Description Payment Pay Period 11/1 - 11/15 2775. 37 Pay Period 11/16 11/30 2775. 36 Pay Period 12/1 - 12/15 2775. 37 Pay Period 12/16 12/31 2775. 36 11101. 4 6 Other Direct Cost Total Other Direct Cost Indirect Cost 0 11/13/2013 Fedex 11/21/2013 Carolina 12/5/2013 Carolina 12/7/2013 Thermo Scientific 12/13/2013 Grainger 12/17/2013 Airgas Postage Plastic Pippets Glass Screw-Cap Vials Phusion RT PCR Kits Chemical Buffer Solution Oxygen 20 CU FT 43. 7 535 526. 25 1545 155. 8 1069. 44 3875. 19 11/13/2013 Fisher Scientific Standard Mini-Centrifuge 1445. 15 11/30/2013 12/31/2013 Indirect Cost Total Indirect Cost 4721. 17 5357. 48 10078. 6 5 Total Costs - 10011338 30235. 8 3 40

Invoice Process: High Risk Monitoring Discrepancies � How to handle discrepancies identified during invoice reviews: ◦ Must be resolved prior to payment ◦ Partial payments of invoices are allowable (e. g. , withholding questioned costs) ◦ Significant discrepancies may indicate the need for a 100% desk audit of the specific invoice or all invoices depending on the nature of the concern ◦ Resolutions must be formally documented on the invoice or payment certification form ◦ At discretion of departments, results may dictate future invoicing requirements (e. g. , submission of timesheets with every invoice) 41

Goods Receipts � � Goods Receipts (GR) are confirmations that goods or services have been received. A GR must be completed for each invoice that is submitted to AP for payment. Goods Receipts facilitate the “Three Way Match”, which means that the P. O. , the Goods Receipt, and the vendor invoice all show the same price and quantity. � A GR request is sent to the ORA STM’s ECC inboxes, who forwards it to the ECC inbox of the appropriate departmental contact. � The Subawards Manager is able to reverse GR’s that were done through the ECC inbox. The dept contact is able to reverse GR’s that were done through Central Receiving. � Two methods for completing goods receipts (see handout). (Central Receiving SAP Role: ZESC_SC_RCV_CNT_All) � � SAP manuals available at: http: //ssc. jhmi. edu/supplychain/training GRs can be reversed by the dept if it was completed via Central Receiving or by the Subawards Manager if not 42

Its taking too long! Can I pay the subsite without the fully executed Subaward? � Complicating factors in completing a sub: ◦ Subsite wants indemnification from sponsor ◦ Subsite has not obtained IRB approval ◦ Sponsor has to approve something- like the replacement of a project director. ◦ Payment/Purchase Order issues �Please do NOT: ◦ Make payments to subsites via online check requests ◦ Provide subsites with purchase order numbers before original contracts are fully-executed. ◦ Create goods receipts before the invoice has been posted in SAP. 43

Troubleshooting… �Generally speaking, the Subs group cannot resolve BW and Real Time Grant Report issues (incorrect encumbrances, etc. ). �We only close the PO/SC, and reverse GRs. �For encumbrance issues contact Purchasing, PO Maintenance, or SPSS accordingly. 44

� If In General… you have questions about what you need to submit or something the subsite is asking for, please call or e-mail us. Subawards Manager 443 -287 -0701 msnow 1@jhmi. edu Michelle Sloan, M. H. A. Subawards Associate 410 -614 -1716 msloan 7@jhmi. edu Rhanota Edwards, M. S. Subawards Specialist 410 -502 -7349 redwar 20@jhmi. edu Jessica Spielman, B. S. Subawards Specialist 410 -502 -0776 jspielman@jhmi. edu Subawards Specialist 410 -502 -0558 pstuart 1@jhmi. edu Melody Snow, M. H. S. Paul Stuart, B. S. 45
 Johns hopkins medicine strategic plan
Johns hopkins medicine strategic plan Johns hopkins medicine strategic plan
Johns hopkins medicine strategic plan Eunice johns and charlie johns
Eunice johns and charlie johns Wilmer eye institute
Wilmer eye institute Dr petri lupus center
Dr petri lupus center Johns hopkins powerpoint template
Johns hopkins powerpoint template Johns hopkins essays that worked
Johns hopkins essays that worked John hopkins model
John hopkins model Johns hopkins community physicians
Johns hopkins community physicians Ictr redcap
Ictr redcap Johns hopkins
Johns hopkins Johns hopkins applied physics lab internship
Johns hopkins applied physics lab internship John hopkins university covid 19 map
John hopkins university covid 19 map John hopkins university
John hopkins university Mercer medical library
Mercer medical library Eschool st johns county
Eschool st johns county Hopkins north junior high school
Hopkins north junior high school Universitatea de medicina si farmacie victor babes
Universitatea de medicina si farmacie victor babes Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine Semmelweis
Semmelweis Lincoln memorial university college of veterinary medicine
Lincoln memorial university college of veterinary medicine King faisal university college of medicine
King faisal university college of medicine King saud university college of medicine
King saud university college of medicine Prodofol
Prodofol King abdulaziz university faculty of medicine
King abdulaziz university faculty of medicine University of wisconsin integrative medicine anxiety
University of wisconsin integrative medicine anxiety Victor babeş university of medicine and pharmacy
Victor babeş university of medicine and pharmacy Victor babes university
Victor babes university Agnes csaki semmelweis
Agnes csaki semmelweis King saud university college of medicine
King saud university college of medicine Faculty of veterinary medicine cairo university logo
Faculty of veterinary medicine cairo university logo Veterinary university brno
Veterinary university brno Slu family medicine residency
Slu family medicine residency Hacettepe university faculty of medicine
Hacettepe university faculty of medicine Al mustansiriya university college of medicine
Al mustansiriya university college of medicine Dosrsal
Dosrsal Semmelweis university faculty of medicine
Semmelweis university faculty of medicine Midwestern university college of dental medicine
Midwestern university college of dental medicine Seoul university medical school
Seoul university medical school University of kentucky college of medicine
University of kentucky college of medicine Faculty of veterinary medicine cairo university
Faculty of veterinary medicine cairo university Unm internal medicine residents
Unm internal medicine residents University of arizona emergency medicine
University of arizona emergency medicine Cairo university faculty of veterinary medicine
Cairo university faculty of veterinary medicine Universidad saint johns
Universidad saint johns Papa johns mision y vision
Papa johns mision y vision