The Increasing Use of Targeted Therapies for Leukemia





- Slides: 20

The Increasing Use of Targeted Therapies for Leukemia and Lymphoma Shira Dinner, MD Northwestern University

Educational Objectives • Define terminology used regarding leukemia • Describe the latest developments in the treatment of acute lymphoblastic leukemia (ALL) - Blinatumomab - Inotuzumab ozogamicin - Chimeric antigen receptor T cells • Engage patients and caregivers in clinical trial discussions on emerging therapies in ALL • Identify strategies fro optimal patient care - What is the best treatment for me?

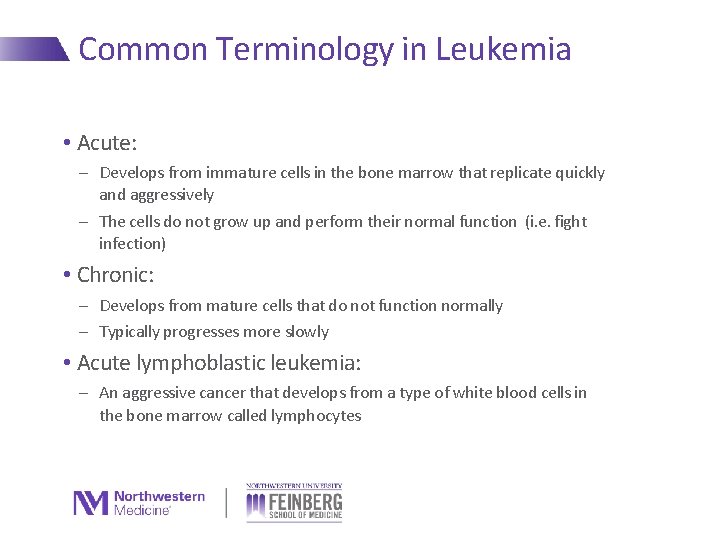
Common Terminology in Leukemia • Acute: - Develops from immature cells in the bone marrow that replicate quickly and aggressively - The cells do not grow up and perform their normal function (i. e. fight infection) • Chronic: - Develops from mature cells that do not function normally - Typically progresses more slowly • Acute lymphoblastic leukemia: - An aggressive cancer that develops from a type of white blood cells in the bone marrow called lymphocytes

Common Terminology in Leukemia • Complete remission (CR) - The absence of any signs or symptoms of the disease - < 5% blast cells in the bone marrow (when looking at the cells under a microscope) and no evidence of leukemia anywhere outside of the bone marrow • Minimal residual disease (MRD) - A very low level of leukemia cells that remain in the bone marrow that cannot be seen by the microscope - Detected by looking for certain gene or chromosome mutations that may be present in the leukemia or by looking at antigens (proteins) on the surface of the leukemia cells • Refractory disease - The leukemia does not respond to treatment or does not achieve a CR • Relapsed disease - The leukemia was in CR, but has now come back

Common Terminology in Leukemia • Overall survival (OS) - The length of time from either the date of diagnosis or the start of treatment for a disease that patients diagnosed are still alive. - In a clinical trial, measuring the overall survival is one way to see how well a new treatment works. • Progression free survival - The length of time during and after the treatment of a disease that the disease remains in remission or does not get worse.

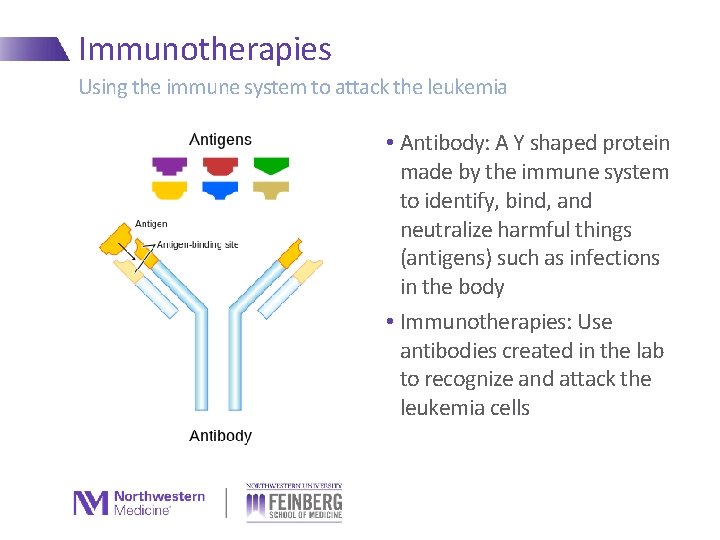
Immunotherapies Using the immune system to attack the leukemia • Antibody: A Y shaped protein made by the immune system to identify, bind, and neutralize harmful things (antigens) such as infections in the body • Immunotherapies: Use antibodies created in the lab to recognize and attack the leukemia cells

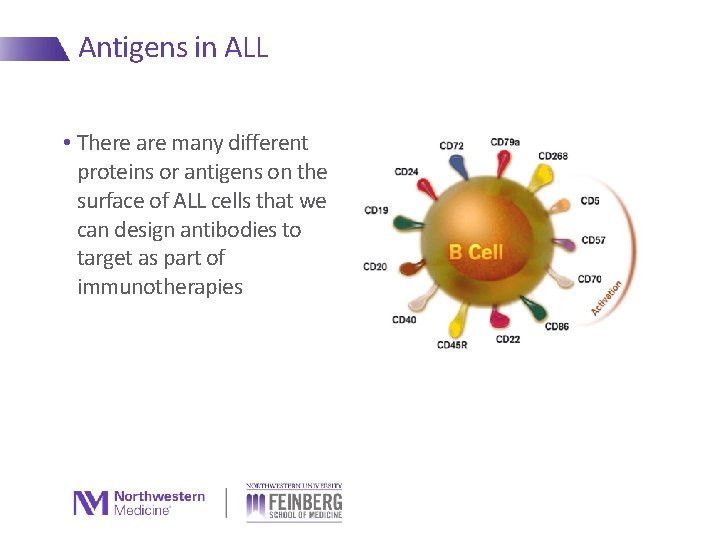
Antigens in ALL • There are many different proteins or antigens on the surface of ALL cells that we can design antibodies to target as part of immunotherapies

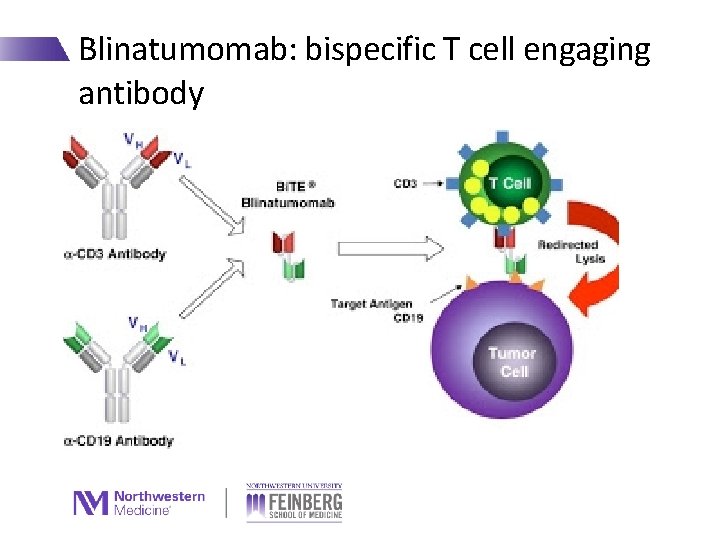
Blinatumomab: bispecific T cell engaging antibody

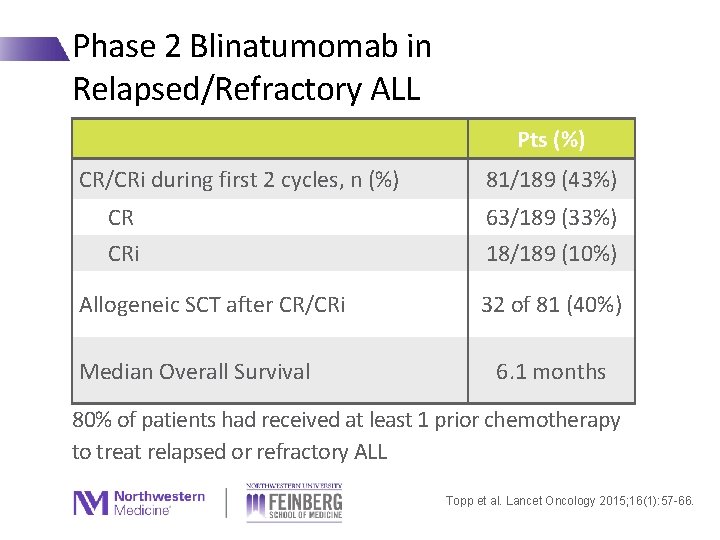
Phase 2 Blinatumomab in Relapsed/Refractory ALL Pts (%) CR/CRi during first 2 cycles, n (%) CR CRi Allogeneic SCT after CR/CRi Median Overall Survival 81/189 (43%) 63/189 (33%) 18/189 (10%) 32 of 81 (40%) 6. 1 months 80% of patients had received at least 1 prior chemotherapy to treat relapsed or refractory ALL Topp et al. Lancet Oncology 2015; 16(1): 57 -66.

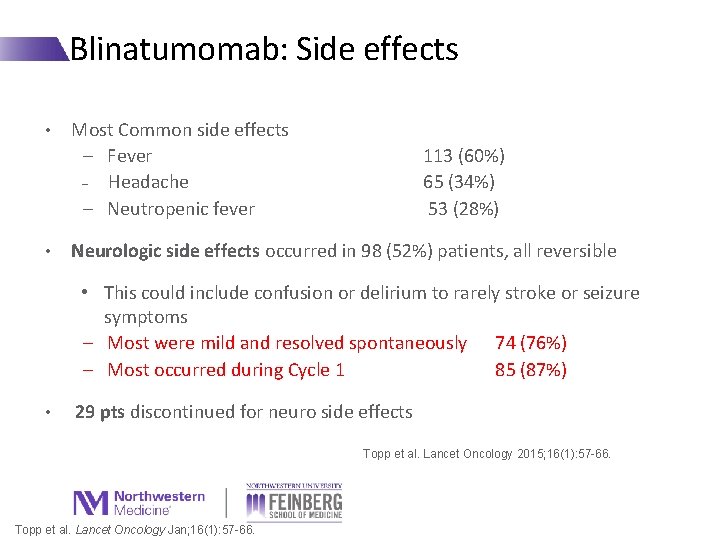
Blinatumomab: Side effects • • Most Common side effects − Fever − Headache − Neutropenic fever 113 (60%) 65 (34%) 53 (28%) Neurologic side effects occurred in 98 (52%) patients, all reversible • This could include confusion or delirium to rarely stroke or seizure symptoms − Most were mild and resolved spontaneously 74 (76%) − Most occurred during Cycle 1 85 (87%) • 29 pts discontinued for neuro side effects Topp et al. Lancet Oncology 2015; 16(1): 57 -66. Topp et al. Lancet Oncology Jan; 16(1): 57 -66.

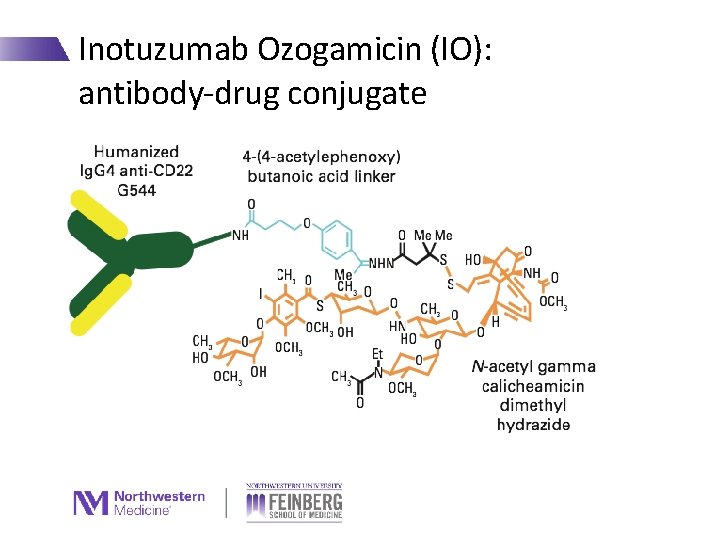
Inotuzumab Ozogamicin (IO): antibody-drug conjugate

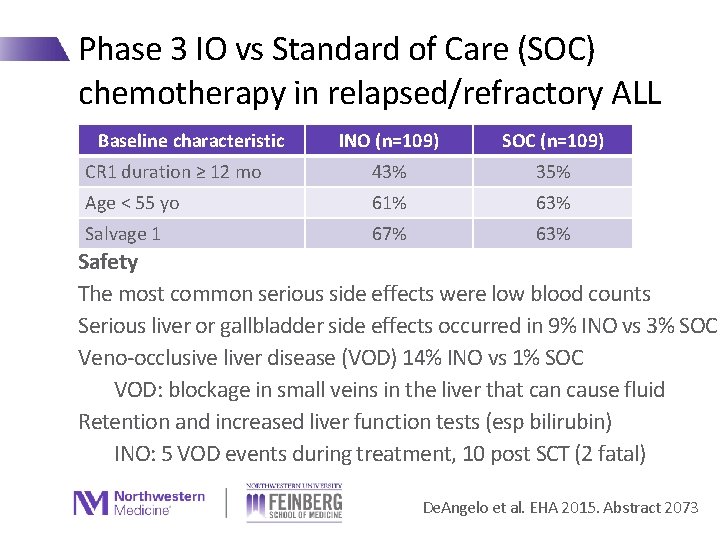
Phase 3 IO vs Standard of Care (SOC) chemotherapy in relapsed/refractory ALL Baseline characteristic INO (n=109) SOC (n=109) CR 1 duration ≥ 12 mo 43% 35% Age < 55 yo 61% 63% Salvage 1 67% 63% Safety The most common serious side effects were low blood counts Serious liver or gallbladder side effects occurred in 9% INO vs 3% SOC Veno-occlusive liver disease (VOD) 14% INO vs 1% SOC VOD: blockage in small veins in the liver that can cause fluid Retention and increased liver function tests (esp bilirubin) INO: 5 VOD events during treatment, 10 post SCT (2 fatal) De. Angelo et al. EHA 2015. Abstract 2073

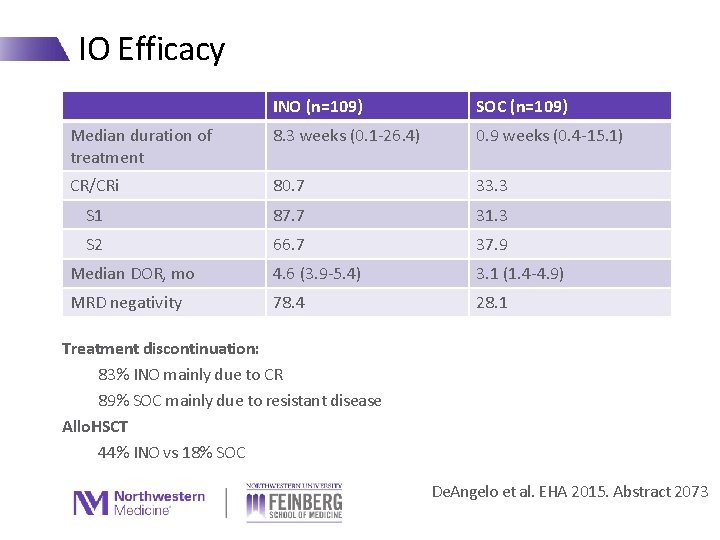
IO Efficacy INO (n=109) SOC (n=109) Median duration of treatment 8. 3 weeks (0. 1 -26. 4) 0. 9 weeks (0. 4 -15. 1) CR/CRi 80. 7 33. 3 S 1 87. 7 31. 3 S 2 66. 7 37. 9 Median DOR, mo 4. 6 (3. 9 -5. 4) 3. 1 (1. 4 -4. 9) MRD negativity 78. 4 28. 1 Treatment discontinuation: 83% INO mainly due to CR 89% SOC mainly due to resistant disease Allo. HSCT 44% INO vs 18% SOC De. Angelo et al. EHA 2015. Abstract 2073

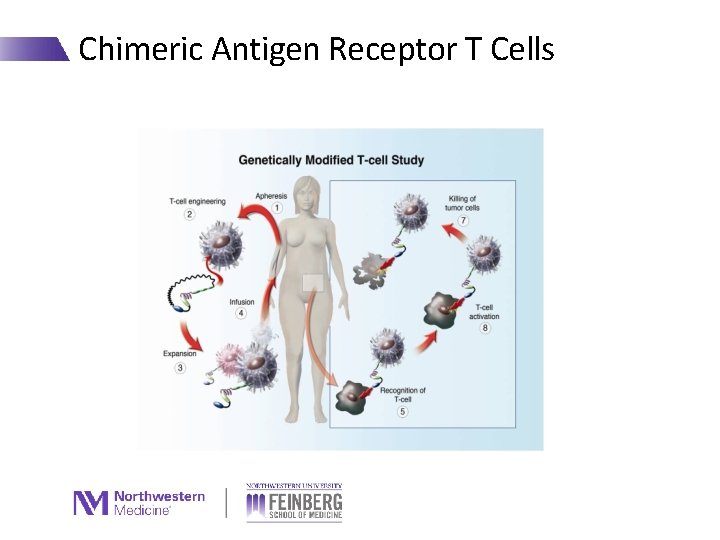
Chimeric Antigen Receptor T Cells

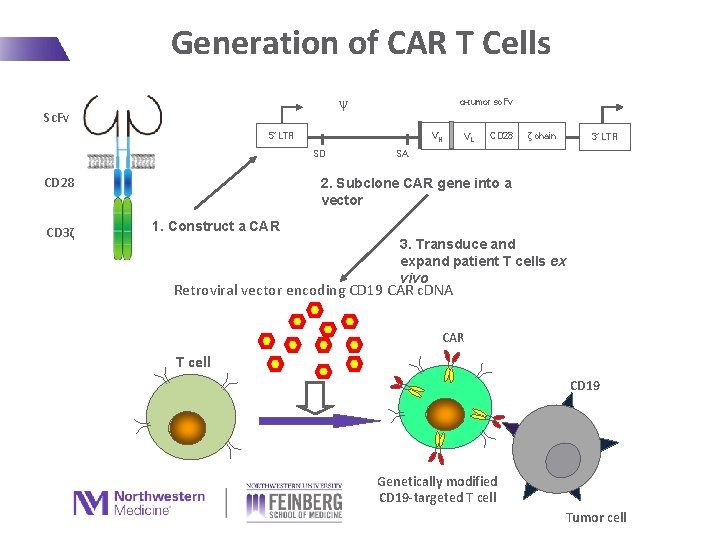
Generation of CAR T Cells ψ Sc. Fv α-tumor sc. Fv 5’ LTR VH SD CD 28 CD 3ζ VL CD 28 ζ chain 3’ LTR SA 2. Subclone CAR gene into a vector 1. Construct a CAR 3. Transduce and expand patient T cells ex vivo Retroviral vector encoding CD 19 CAR c. DNA CAR T cell CD 19 Genetically modified CD 19 -targeted T cell Tumor cell

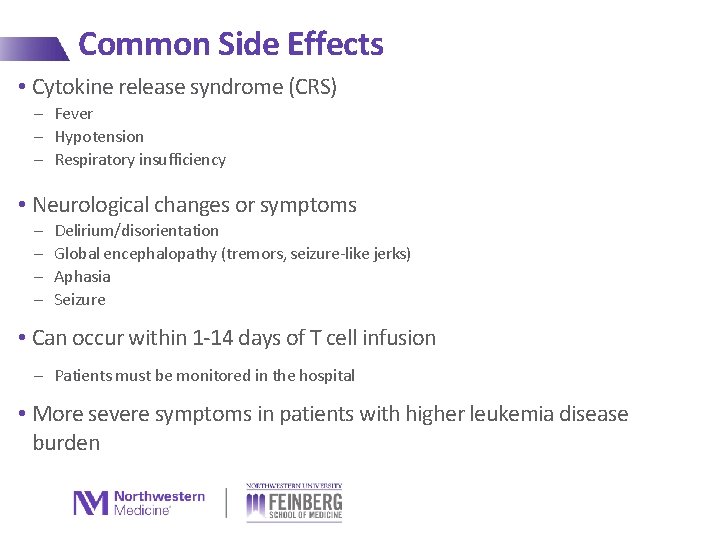
Common Side Effects • Cytokine release syndrome (CRS) - Fever - Hypotension - Respiratory insufficiency • Neurological changes or symptoms - Delirium/disorientation Global encephalopathy (tremors, seizure-like jerks) Aphasia Seizure • Can occur within 1 -14 days of T cell infusion - Patients must be monitored in the hospital • More severe symptoms in patients with higher leukemia disease burden

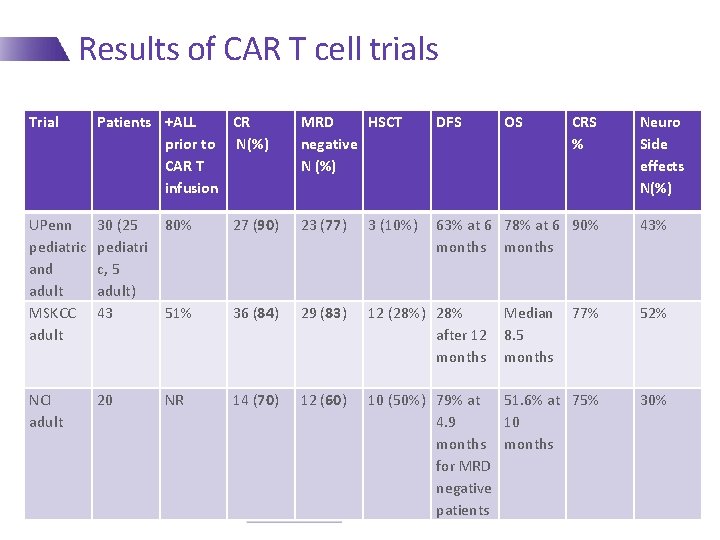
Results of CAR T cell trials Trial Patients +ALL CR prior to N(%) CAR T infusion MRD HSCT negative N (%) DFS OS UPenn pediatric and adult MSKCC adult 30 (25 pediatri c, 5 adult) 43 80% 27 (90) 23 (77) 3 (10%) 63% at 6 78% at 6 90% months 51% 36 (84) 29 (83) 12 (28%) 28% after 12 months 77% 52% NCI adult 20 NR 14 (70) 12 (60) 10 (50%) 79% at 51. 6% at 75% 4. 9 10 months for MRD negative patients 30% Median 8. 5 months CRS % Neuro Side effects N(%) 43%

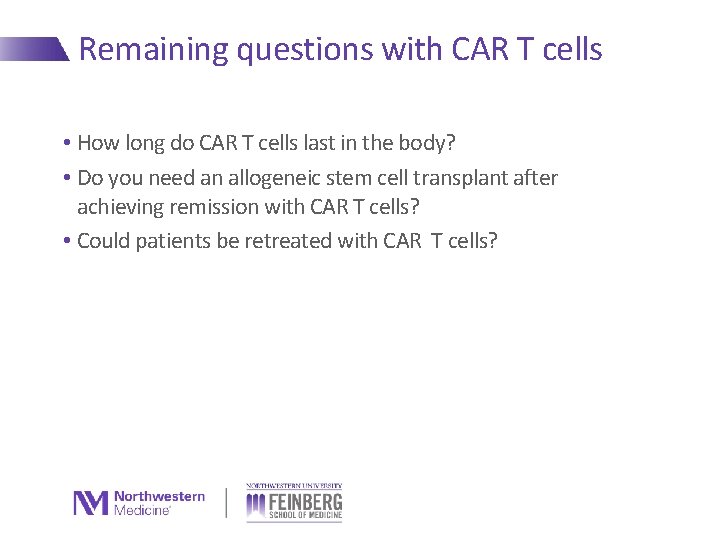
Remaining questions with CAR T cells • How long do CAR T cells last in the body? • Do you need an allogeneic stem cell transplant after achieving remission with CAR T cells? • Could patients be retreated with CAR T cells?

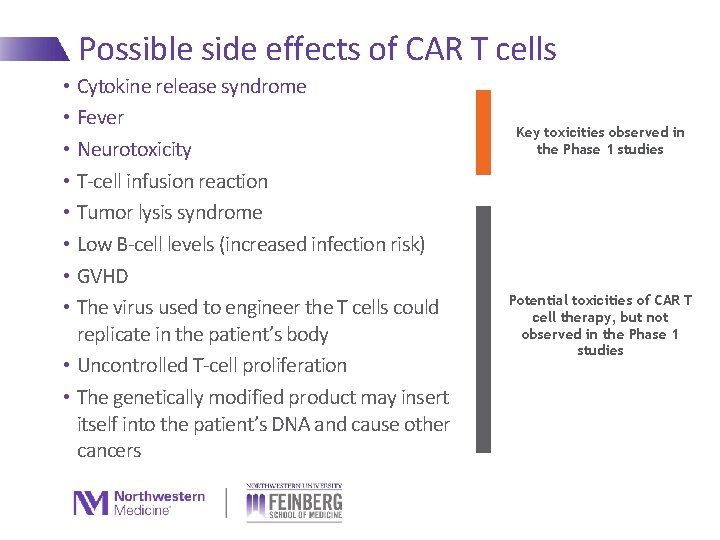
Possible side effects of CAR T cells • Cytokine release syndrome • Fever • Neurotoxicity • T-cell infusion reaction • Tumor lysis syndrome • Low B-cell levels (increased infection risk) • GVHD • The virus used to engineer the T cells could replicate in the patient’s body • Uncontrolled T-cell proliferation • The genetically modified product may insert itself into the patient’s DNA and cause other cancers Key toxicities observed in the Phase 1 studies Potential toxicities of CAR T cell therapy, but not observed in the Phase 1 studies 19

Summary • New therapies for leukemia and lymphoma are taking advantage of copying or utilizing the immune system to attack the cancer • Several new immunotherapies are available for ALL - No clinical trials have compared blinatumomab vs IO vs CAR T cells - We do not yet know which is the “best” treatment or what order we should give the treatments in - It is important to consider side effects when selecting a treatment with your doctor • Blinatumomab: neurologic side effects, cytokine release syndrome • IO: liver side effects • CAR T cells: CRS, neurologic side effects • Talk to your doctor about a clinical trial
 Psychodynamic vs psychoanalytic
Psychodynamic vs psychoanalytic Zhang wang leukemia
Zhang wang leukemia Chronic myeloid leukemia
Chronic myeloid leukemia Chronic myeloid leukemia treatment
Chronic myeloid leukemia treatment Conclusion of leukemia
Conclusion of leukemia Lymphoproliferative disease
Lymphoproliferative disease Nk leukemia
Nk leukemia Leukemia vs lymphoma
Leukemia vs lymphoma Leukemia survival rate
Leukemia survival rate Leukemia statics
Leukemia statics Leukemia
Leukemia Hairy cell leukemia
Hairy cell leukemia Acute mylogenous leukemia
Acute mylogenous leukemia Tomy a suffix denoting an incision
Tomy a suffix denoting an incision Differentiation syndrome
Differentiation syndrome Chronic myeloid leukemia
Chronic myeloid leukemia Leukemia aleukemik adalah
Leukemia aleukemik adalah Lll leukemia
Lll leukemia Leukemia statics
Leukemia statics Suffix -ion medical terminology
Suffix -ion medical terminology Chronic myeloid leukemia
Chronic myeloid leukemia