The Earths Resources Natural and synthetic items Synthetic

























































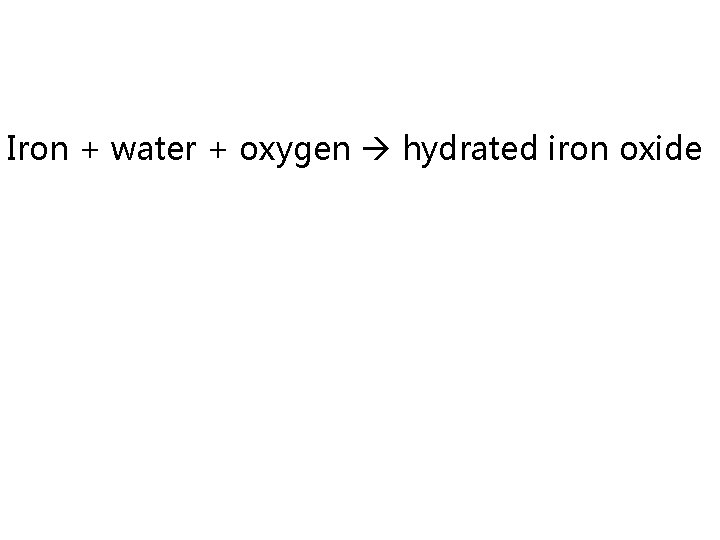













- Slides: 79

The Earth’s Resources


Natural and synthetic items

Synthetic replacements • Common examples: – Wool for use in clothing is now being replaced by acrylic fibre – Cotton for use in clothing is now being replaced by polyester – Wood for use in construction is now being replaced by PVC (a plastic) and MDF composites



• Resulting need for sustainable development



Water safe to drink



Water is not always safe to drink: • High concentrations of salts • Microbes

Potable water

To obtain potable water • Choose a source of water • Filter to remove large objects • Sterilise (kill microbes) – Chlorine – Ozone – UV light


Desalination

• Can be carried out by: – Distillation – Reverse osmosis using membranes • Requires a lot of energy


• 17 -24

Treating Waste Water

• Sewage • Agricultural waste water • Industrial waste water



• Sewage and agricultural waste water can have – Organic matter – Harmful microbes • Industrial waste can have – Organic matter – Harmful chemicals

• https: //www. youtube. com/watch? v=xy. U 34 Fh i 0 FY

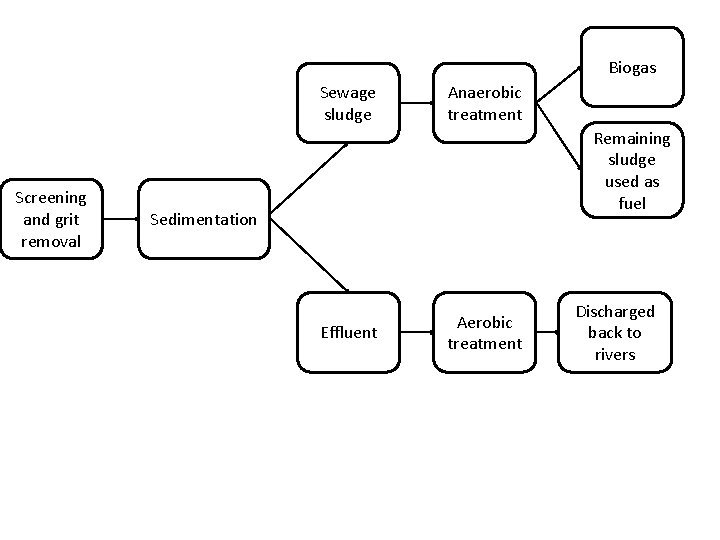
Biogas Sewage sludge Screening and grit removal Anaerobic treatment Remaining sludge used as fuel Sedimentation Effluent Aerobic treatment Discharged back to rivers

• Steps: – Screening and grit removal – Sedimentation to produce sewage sludge and effluent – Anaerobic digestion of sewage sludge • Biogas produced • Remaining sludge can be used as fuel – Aerobic biological treatment of effluent • Effluent can now be discharged back into rivers

• 25 -34

Life Cycle Assessment and Recycling

Importance of LCA • https: //www. youtube. com/watch? v=xq. Y 8 FUD c. ATE

Stages of LCA • • Extracting and processing raw materials Manufacturing and packaging Use and operation during its lifetime Disposal at the end of lifetime (including transport and distribution)

Plastic or paper bags?


Reduce, Reuse, Recycle

The problem

Reduce, Reuse, Recycle

• Resources are limited (non-renewable) • Environmental impacts of quarrying – Habitat loss – Generation of CO 2 – Noise pollution – Generation of hazardous waste

• Reduce: To simply use less • Reuse: to use again • Recycle: to use manufacturing processes to make new products • Case study: plastic bags in the UK






• What happens to the plastic you throw away? • https: //www. youtube. com/watch? v=_6 xl. Ny. W Pp. B 8

Plastic bag charge • Plastic bag use dropped by 85%

Glass bottles • Can be reused • Can be crushed and recycled to make new glass products


• Recycling: https: //www. youtube. com/watch? v=b 7 GMpjx 2 j. DQ

• 36 -46

Metals • Can be recycled by melting and recasting • Need to be separated first which can cause cost

Alternative methods of extracting metals

• The problem: – The Earth’s resources of metal are finite – Digging, moving and disposing of the large amounts of rock for traditional mining is problematic • Habitat loss • Greenhouse gas emissions

Phytomining (copper) 1. Grow plants near/on metal compounds 2. Harvest plants 3. Burn plants 4. Ash contains the metal compound (carbon neutral? )

Bioleaching (copper) 1. Grow bacteria near/on metal compound 2. Bacteria produce leachate solutions that have metal compound

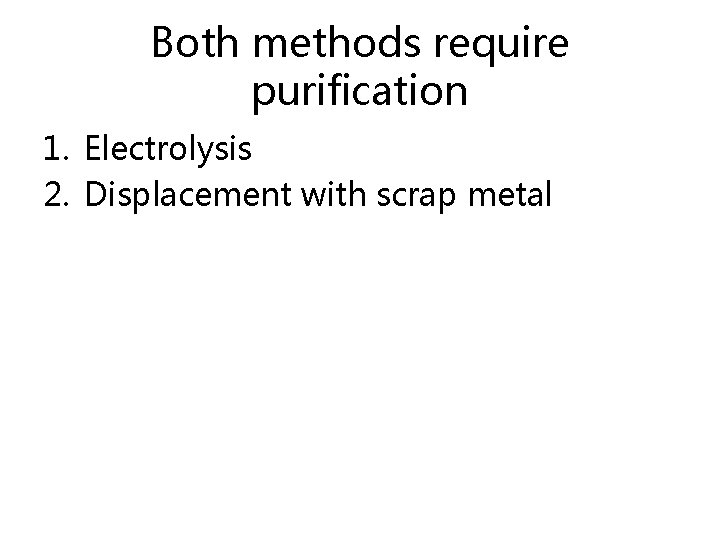
Both methods require purification 1. Electrolysis 2. Displacement with scrap metal

• End of unit • Q 47 -54

Using Materials (Triple only)

Corrosion
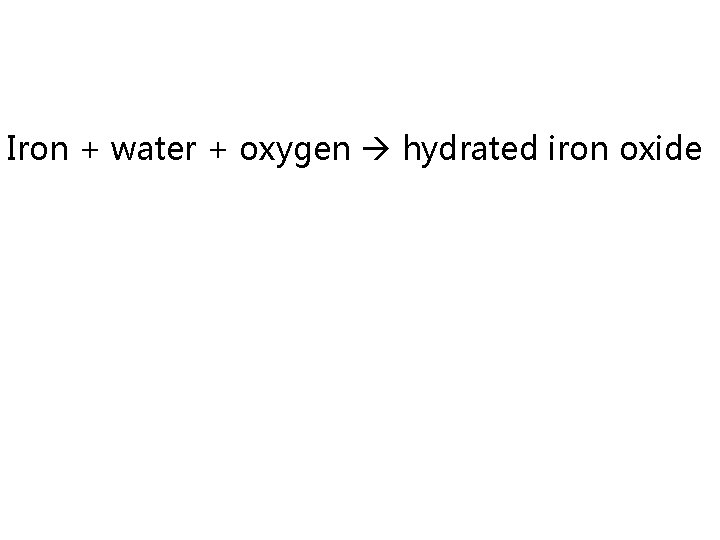
Iron + water + oxygen hydrated iron oxide

How to protect metals from corrosion • Coatings – Grease – Paint – Electroplate • “Natural” coatings (aluminium oxide) • Sacrificial protections


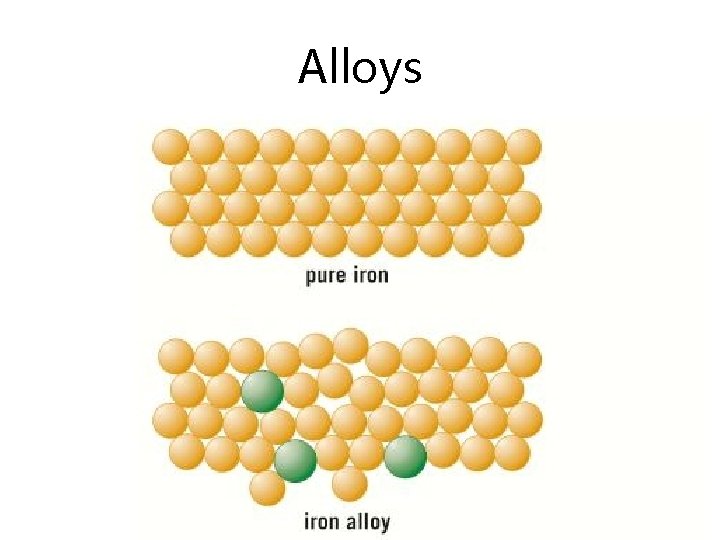
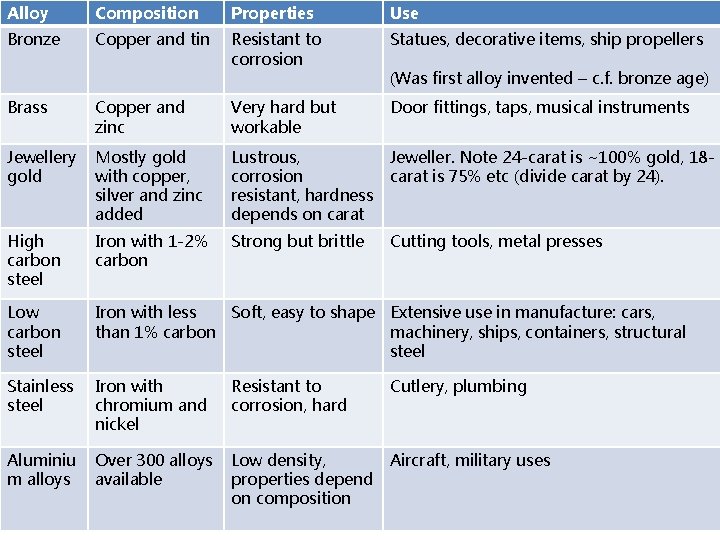
Alloys

Alloy Composition Properties Use Bronze Copper and tin Resistant to corrosion Statues, decorative items, ship propellers (Was first alloy invented – c. f. bronze age) Brass Copper and zinc Very hard but workable Door fittings, taps, musical instruments Jewellery gold Mostly gold with copper, silver and zinc added Lustrous, corrosion resistant, hardness depends on carat Jeweller. Note 24 -carat is ~100% gold, 18 carat is 75% etc (divide carat by 24). High carbon steel Iron with 1 -2% carbon Strong but brittle Cutting tools, metal presses Low carbon steel Iron with less Soft, easy to shape Extensive use in manufacture: cars, than 1% carbon machinery, ships, containers, structural steel Stainless steel Iron with chromium and nickel Resistant to corrosion, hard Cutlery, plumbing Aluminiu m alloys Over 300 alloys available Low density, properties depend on composition Aircraft, military uses

Students should be able to: • recall a use of each of the alloys specified • interpret and evaluate the composition and uses of alloys other than those specified given appropriate information.

• Q 17 -30

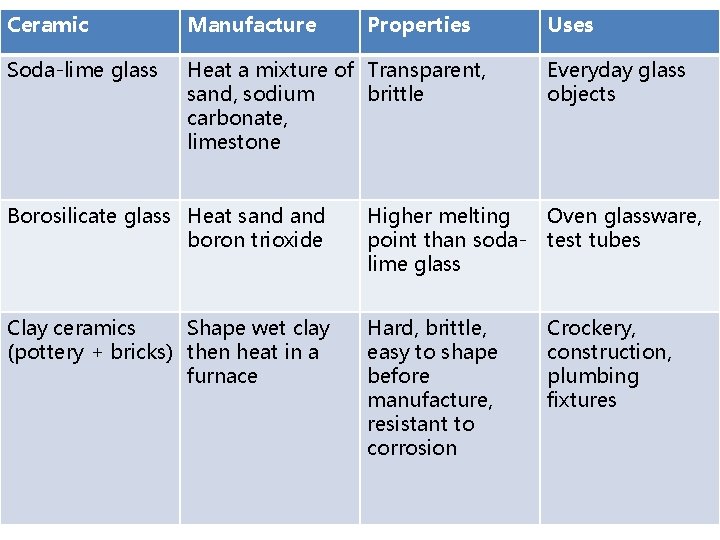
Ceramics

Ceramic Manufacture Properties Soda-lime glass Heat a mixture of Transparent, sand, sodium brittle carbonate, limestone Uses Everyday glass objects Borosilicate glass Heat sand boron trioxide Higher melting point than sodalime glass Oven glassware, test tubes Clay ceramics Shape wet clay (pottery + bricks) then heat in a furnace Hard, brittle, easy to shape before manufacture, resistant to corrosion Crockery, construction, plumbing fixtures

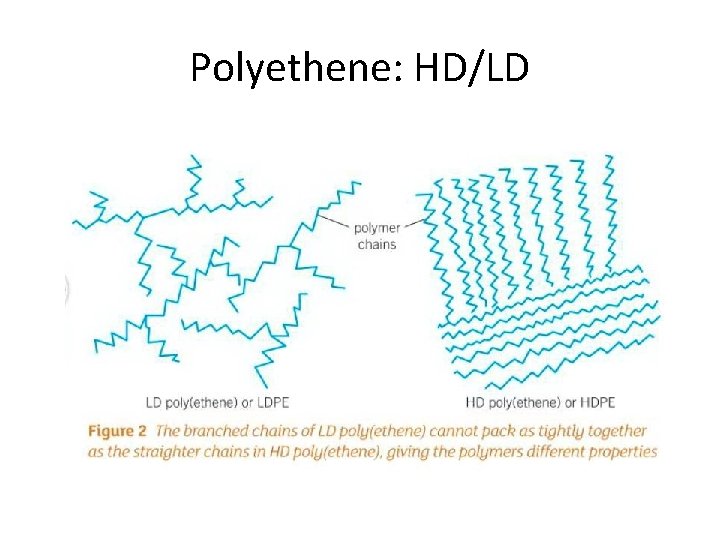
Polyethene: HD/LD

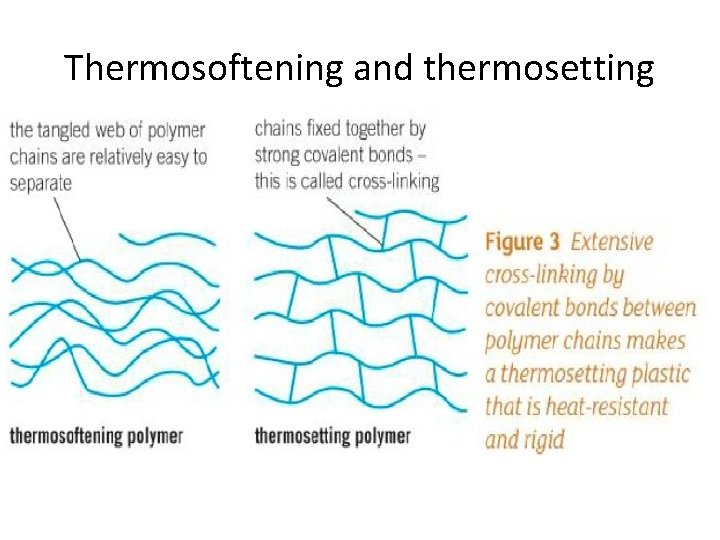
Thermosoftening and thermosetting

• 31 -41

Composites

• Composites are mixtures of materials for specific uses – The main material is called the matrix or binder – The second material is usually added as threads or fragments • Examples: – – – Concrete (cement, sand gravel) Reinforced concrete (concrete + steel rods) Plywood (thin sheets of wood and glue) MDF (whoodchips or shavings in a polymer resin) Pykrete (ice and sawdust)

• 44 -46

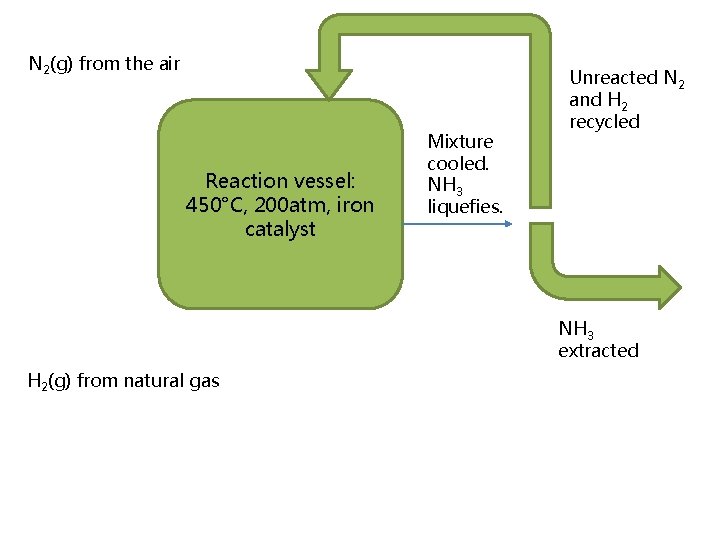
The Haber Process

N 2(g) from the air Reaction vessel: 450°C, 200 atm, iron catalyst Mixture cooled. NH 3 liquefies. Unreacted N 2 and H 2 recycled NH 3 extracted H 2(g) from natural gas

• N 2(g) + 3 H 2(g) ⇌ 2 NH 3(g) • Effects on yield and rate – Temperature (forward reaction is exothermic) – Pressure • “compromise” conditions

NPK Fertilisers

NPK Fertilisers • Nitrogen – From ammonia – Used to manufacture ammonium salts and nitric acid • Phosphorous – Comes from mined phosphate rock [Ca 3(PO 4)2] – Treat the rock with nitric or sulphuric acid • Potassium – Potassium chloride and potassium sulphate common sources – Obtained by mining
 Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Acuity charting forms
Acuity charting forms Lliver
Lliver Mosby items and derived items
Mosby items and derived items Reproductive system
Reproductive system Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Absorptive atelectasis
Absorptive atelectasis Absorption atelectasis
Absorption atelectasis Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Mosby items and derived items
Mosby items and derived items Difference between transforming and transformed resources
Difference between transforming and transformed resources Fixed resources examples
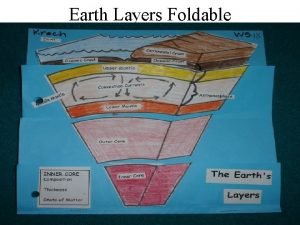
Fixed resources examples The earths layer foldable
The earths layer foldable Earths roation
Earths roation Whats earths moon called
Whats earths moon called What makes one biome different from another?
What makes one biome different from another? Most abundant element in earth's crust
Most abundant element in earth's crust Plasticity in earth's layers
Plasticity in earth's layers Whats earths moon called
Whats earths moon called Whats the thickest layer of the earth
Whats the thickest layer of the earth Earths early atmosphere contained
Earths early atmosphere contained Earth's layer foldable
Earth's layer foldable Earths major crustal plates
Earths major crustal plates Earths orbit seasons
Earths orbit seasons Brown earth soil profile
Brown earth soil profile Earths physical features
Earths physical features Honey as fertilizer for plants
Honey as fertilizer for plants Whats the name of earths moon
Whats the name of earths moon Pangea explanation
Pangea explanation Earths crust
Earths crust Earths interior
Earths interior What does the earths tilt cause
What does the earths tilt cause Layers of the atmosphere
Layers of the atmosphere What shape is the earths orbit
What shape is the earths orbit The emperor constantine i recycled sculpture
The emperor constantine i recycled sculpture What does earths tilt do
What does earths tilt do Earths boundaries
Earths boundaries 4 spheres of the earth
4 spheres of the earth Plant assets are
Plant assets are Some natural resources such as wheat and cattle are
Some natural resources such as wheat and cattle are Landforms and natural resources
Landforms and natural resources Natural resources from landforms
Natural resources from landforms Accumulated depreciation straight line method
Accumulated depreciation straight line method Natural resources and population growth
Natural resources and population growth Natural resources and uses
Natural resources and uses Problems of water resources
Problems of water resources Agriculture abbreviation
Agriculture abbreviation Plant assets, natural resources, and intangible assets
Plant assets, natural resources, and intangible assets Renewable vs nonrenewable resources worksheet
Renewable vs nonrenewable resources worksheet Natural capital and natural income
Natural capital and natural income Australia major natural resources
Australia major natural resources Central australia natural resources
Central australia natural resources What are types of natural resources
What are types of natural resources Natural resources vocabulary
Natural resources vocabulary Resources of the northeast region
Resources of the northeast region Russia resources
Russia resources Natural resources in southeast asia
Natural resources in southeast asia Southwest asia natural resources
Southwest asia natural resources What is michigan's natural resources
What is michigan's natural resources What are cuba's natural resources
What are cuba's natural resources Natural resources in west region
Natural resources in west region Landform regions map of canada
Landform regions map of canada Australia natural resources
Australia natural resources