The Dipole Moment of the Sulfuric Acid Monomer




- Slides: 23

The Dipole Moment of the Sulfuric Acid Monomer Galen Sedo, Jane Curtis, Kenneth R. Leopold Department of Chemistry, University of Minnesota

Investigating Sulfuric Acid Systems Sulfuric Acid Aerosols • A principle source of sulfate-containing atmospheric particles • High affinity for water and a high rate of nitrogen species uptake Nucleation Theory • Homogeneous and Heterogeneous particle growth • Ion-induced and ion-mediated nucleation theory • Charge-dipole interactions

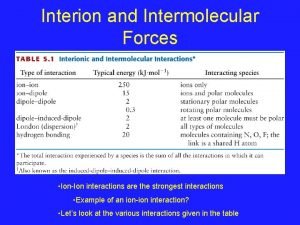
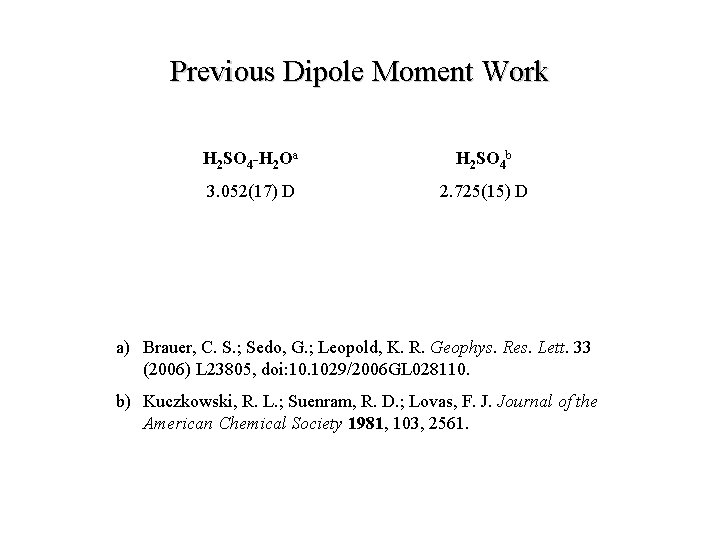
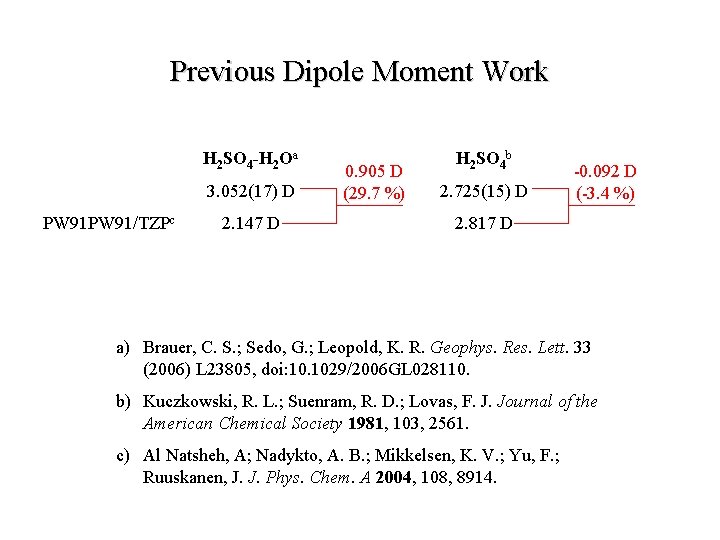
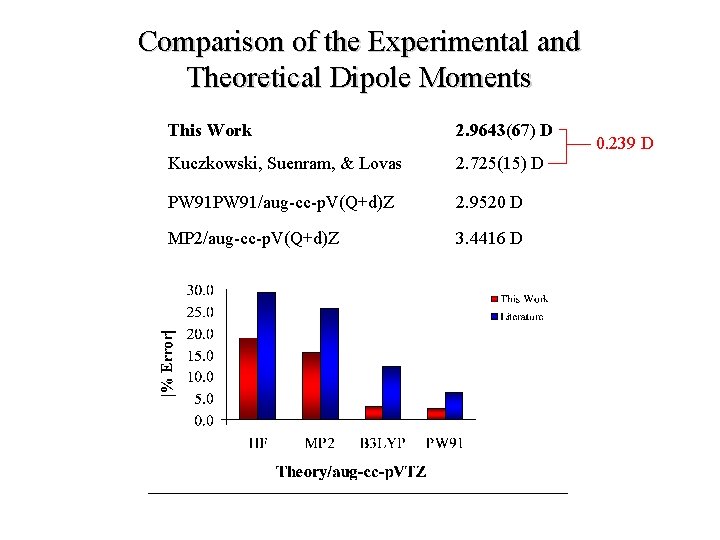
Previous Dipole Moment Work H 2 SO 4 -H 2 Oa H 2 SO 4 b 3. 052(17) D 2. 725(15) D a) Brauer, C. S. ; Sedo, G. ; Leopold, K. R. Geophys. Res. Lett. 33 (2006) L 23805, doi: 10. 1029/2006 GL 028110. b) Kuczkowski, R. L. ; Suenram, R. D. ; Lovas, F. J. Journal of the American Chemical Society 1981, 103, 2561.

Previous Dipole Moment Work H 2 SO 4 -H 2 Oa 3. 052(17) D PW 91/TZPc 2. 147 D 0. 905 D (29. 7 %) H 2 SO 4 b 2. 725(15) D -0. 092 D (-3. 4 %) 2. 817 D a) Brauer, C. S. ; Sedo, G. ; Leopold, K. R. Geophys. Res. Lett. 33 (2006) L 23805, doi: 10. 1029/2006 GL 028110. b) Kuczkowski, R. L. ; Suenram, R. D. ; Lovas, F. J. Journal of the American Chemical Society 1981, 103, 2561. c) Al Natsheh, A; Nadykto, A. B. ; Mikkelsen, K. V. ; Yu, F. ; Ruuskanen, J. J. Phys. Chem. A 2004, 108, 8914.

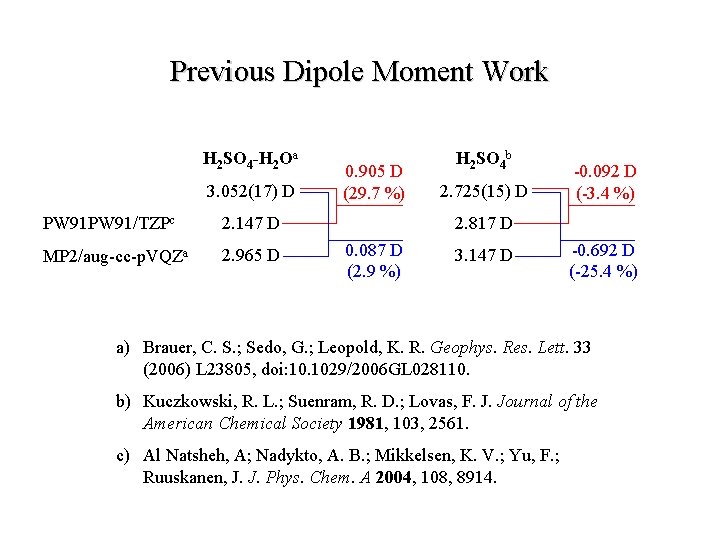
Previous Dipole Moment Work H 2 SO 4 -H 2 Oa 3. 052(17) D PW 91/TZPc 2. 147 D MP 2/aug-cc-p. VQZa 2. 965 D 0. 905 D (29. 7 %) H 2 SO 4 b 2. 725(15) D -0. 092 D (-3. 4 %) 2. 817 D 0. 087 D (2. 9 %) 3. 147 D -0. 692 D (-25. 4 %) a) Brauer, C. S. ; Sedo, G. ; Leopold, K. R. Geophys. Res. Lett. 33 (2006) L 23805, doi: 10. 1029/2006 GL 028110. b) Kuczkowski, R. L. ; Suenram, R. D. ; Lovas, F. J. Journal of the American Chemical Society 1981, 103, 2561. c) Al Natsheh, A; Nadykto, A. B. ; Mikkelsen, K. V. ; Yu, F. ; Ruuskanen, J. J. Phys. Chem. A 2004, 108, 8914.

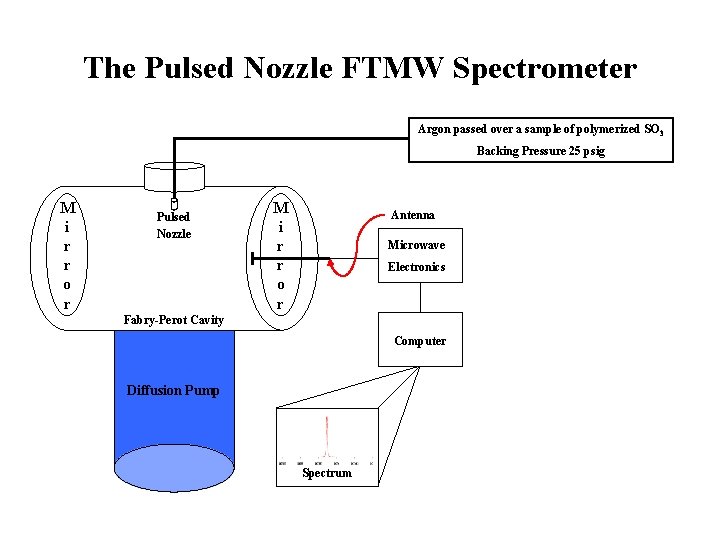
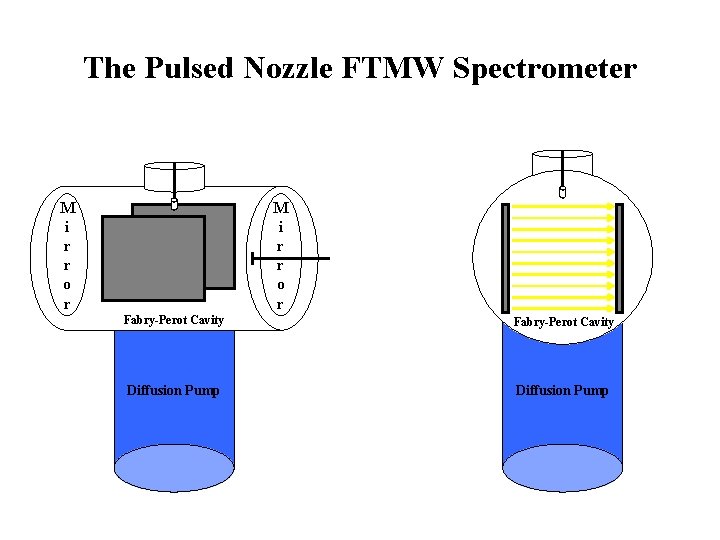
The Pulsed Nozzle FTMW Spectrometer Argon passed over a sample of polymerized SO 3 Backing Pressure 25 psig M i r r o r Pulsed Nozzle M i r r o r Antenna Microwave Electronics Fabry-Perot Cavity Computer Diffusion Pump Spectrum

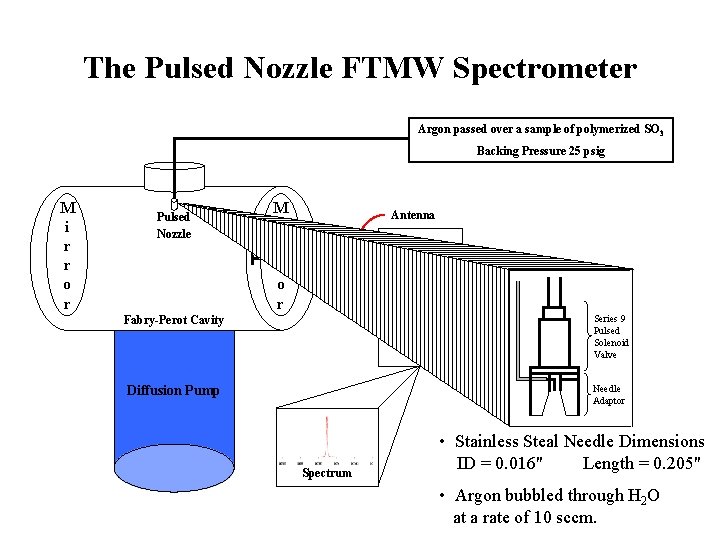
The Pulsed Nozzle FTMW Spectrometer Argon passed over a sample of polymerized SO 3 Backing Pressure 25 psig M i r r o r Pulsed Nozzle M i r r o r Antenna Microwave Electronics Fabry-Perot Cavity Computer Diffusion Pump Series 9 Pulsed Solenoid Valve Needle Adaptor Spectrum • Stainless Steal Needle Dimensions ID = 0. 016" Length = 0. 205" • Argon bubbled through H 2 O at a rate of 10 sccm.

The Pulsed Nozzle FTMW Spectrometer M i r r o r Fabry-Perot Cavity Diffusion Pump

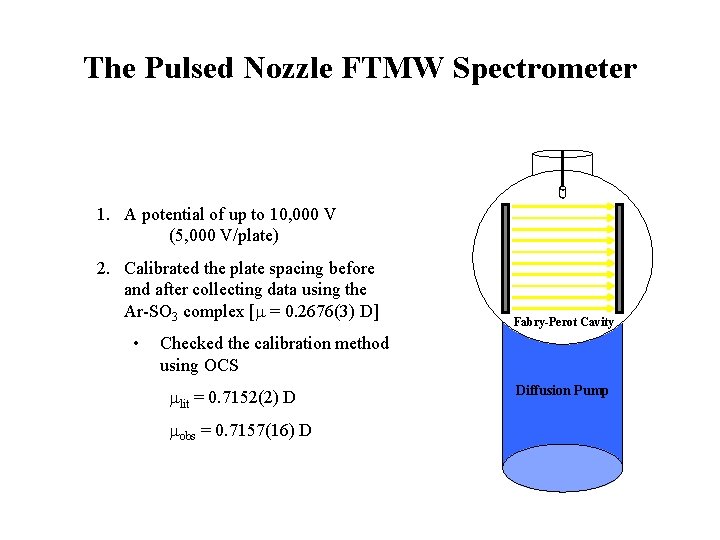
The Pulsed Nozzle FTMW Spectrometer 1. A potential of up to 10, 000 V (5, 000 V/plate) 2. Calibrated the plate spacing before and after collecting data using the Ar-SO 3 complex [m = 0. 2676(3) D] • Fabry-Perot Cavity Checked the calibration method using OCS mlit = 0. 7152(2) D mobs = 0. 7157(16) D Diffusion Pump

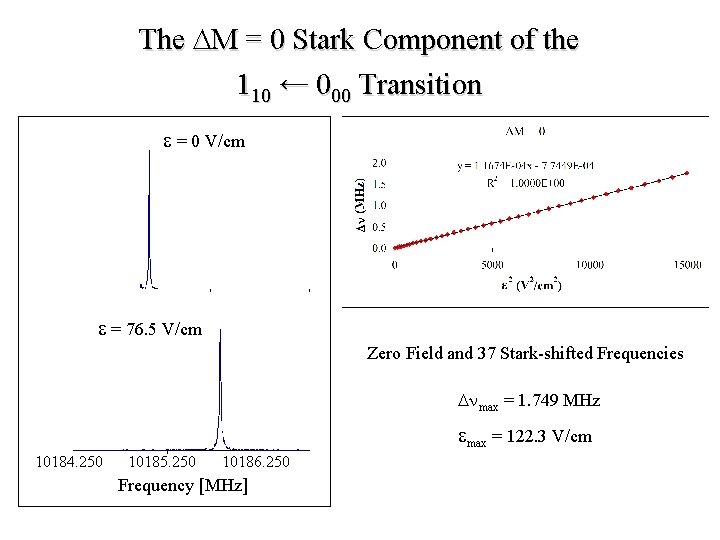
The DM = 0 Stark Component of the 110 ← 000 Transition e = 0 V/cm e = 76. 5 V/cm Zero Field and 37 Stark-shifted Frequencies Dnmax = 1. 749 MHz emax = 122. 3 V/cm 10184. 250 10185. 250 10186. 250 Frequency [MHz]

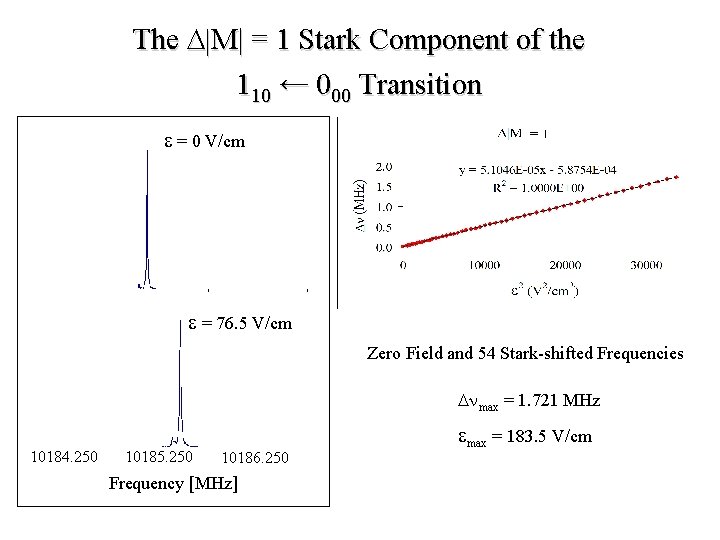
The D|M| = 1 Stark Component of the 110 ← 000 Transition e = 0 V/cm e = 76. 5 V/cm Zero Field and 54 Stark-shifted Frequencies Dnmax = 1. 721 MHz 10184. 250 10185. 250 10186. 250 Frequency [MHz] emax = 183. 5 V/cm

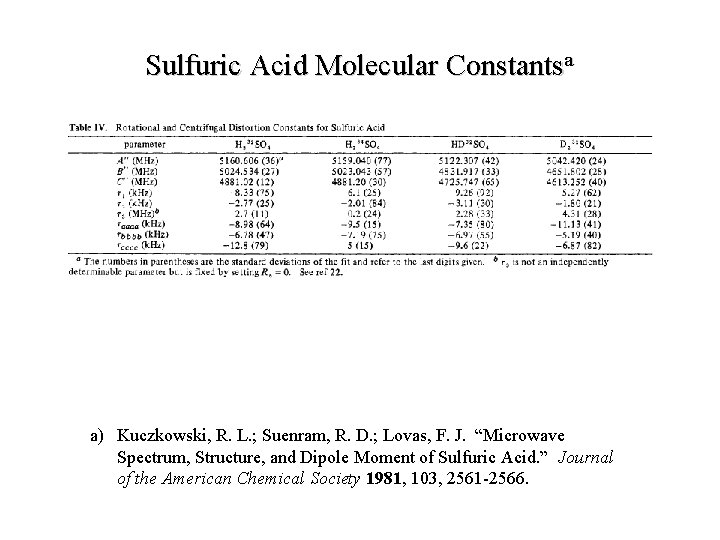
Sulfuric Acid Molecular Constantsa a) Kuczkowski, R. L. ; Suenram, R. D. ; Lovas, F. J. “Microwave Spectrum, Structure, and Dipole Moment of Sulfuric Acid. ” Journal of the American Chemical Society 1981, 103, 2561 -2566.

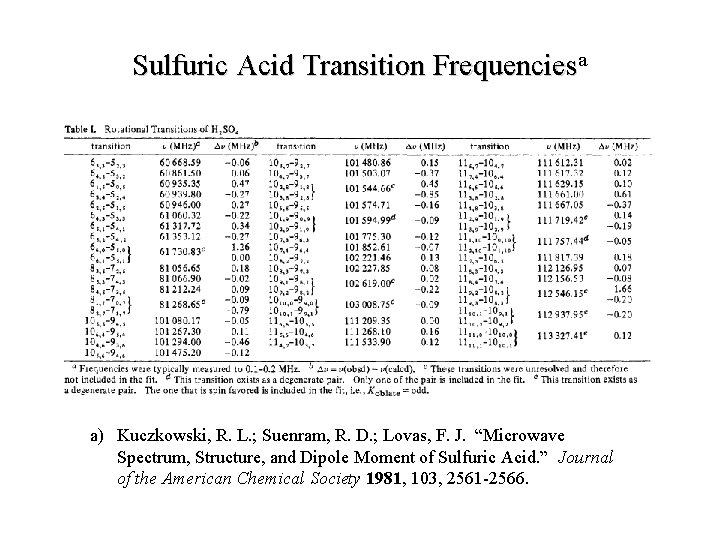
Sulfuric Acid Transition Frequenciesa a) Kuczkowski, R. L. ; Suenram, R. D. ; Lovas, F. J. “Microwave Spectrum, Structure, and Dipole Moment of Sulfuric Acid. ” Journal of the American Chemical Society 1981, 103, 2561 -2566.

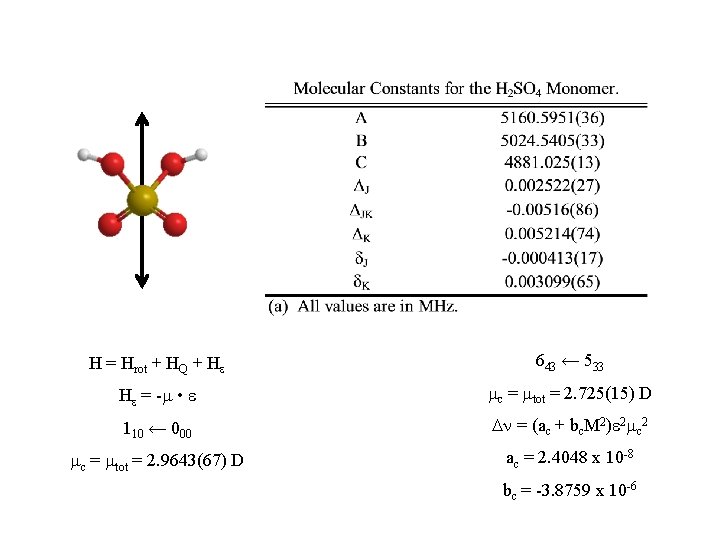
H = Hrot + HQ + He 643 ← 533 He = -m • e mc = mtot = 2. 725(15) D 110 ← 000 Dn = (ac + bc. M 2)e 2 mc 2 mc = mtot = 2. 9643(67) D ac = 2. 4048 x 10 -8 bc = -3. 8759 x 10 -6

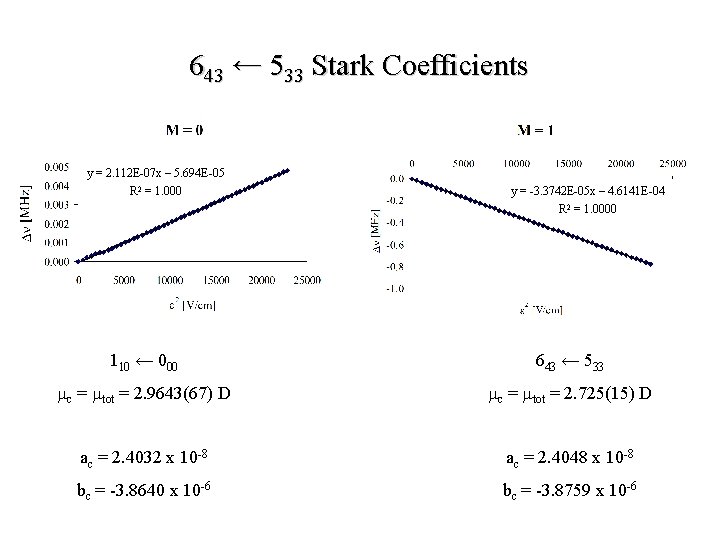
643 ← 533 Stark Coefficients y = 2. 112 E-07 x – 5. 694 E-05 R 2 = 1. 000 y = -3. 3742 E-05 x – 4. 6141 E-04 R 2 = 1. 0000 110 ← 000 643 ← 533 mc = mtot = 2. 9643(67) D mc = mtot = 2. 725(15) D ac = 2. 4032 x 10 -8 ac = 2. 4048 x 10 -8 bc = -3. 8640 x 10 -6 bc = -3. 8759 x 10 -6

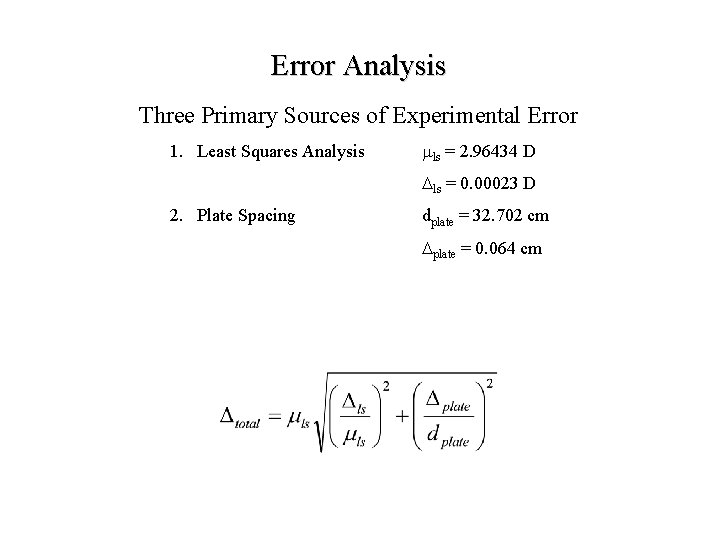
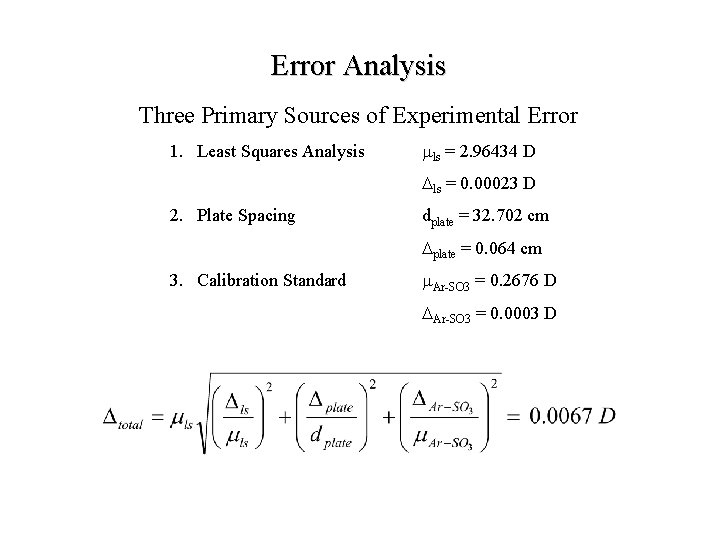
Error Analysis Three Primary Sources of Experimental Error 1. Least Squares Analysis mls = 2. 96434 D Dls = 0. 00023 D

Error Analysis Three Primary Sources of Experimental Error 1. Least Squares Analysis mls = 2. 96434 D Dls = 0. 00023 D 2. Plate Spacing dplate = 32. 702 cm Dplate = 0. 064 cm

Error Analysis Three Primary Sources of Experimental Error 1. Least Squares Analysis mls = 2. 96434 D Dls = 0. 00023 D 2. Plate Spacing dplate = 32. 702 cm Dplate = 0. 064 cm 3. Calibration Standard m. Ar-SO 3 = 0. 2676 D DAr-SO 3 = 0. 0003 D

Comparison of the Experimental and Theoretical Dipole Moments This Work 2. 9643(67) D Kuczkowski, Suenram, & Lovas 2. 725(15) D PW 91/aug-cc-p. V(Q+d)Z 2. 9520 D MP 2/aug-cc-p. V(Q+d)Z 3. 4416 D 0. 239 D

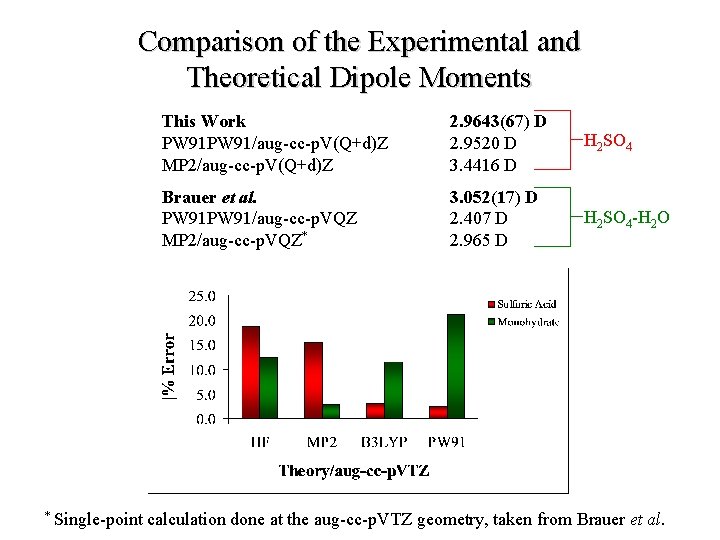
Comparison of the Experimental and Theoretical Dipole Moments * Single-point This Work PW 91/aug-cc-p. V(Q+d)Z MP 2/aug-cc-p. V(Q+d)Z 2. 9643(67) D 2. 9520 D 3. 4416 D H 2 SO 4 Brauer et al. PW 91/aug-cc-p. VQZ MP 2/aug-cc-p. VQZ* 3. 052(17) D 2. 407 D 2. 965 D H 2 SO 4 -H 2 O calculation done at the aug-cc-p. VTZ geometry, taken from Brauer et al.

Conclusions 1. The dipole moment of the sulfuric acid monomer has been refined using Fourier transform microwave spectroscopy. • 91 Stark-shifted frequencies were collect for the 110 ← 000 rotational transition. • The newly measured value, 2. 9643(67) D, represents an increase of approximately 0. 24 D over the previously published value. 2. The dipole moments of the sulfuric acid monomer and mono-hydrate were calculated using both ab initio and Density Functional Theory. • The calculated dipole moments show convergence with increasing basis set size. • The agreement between the measured experimental values and those of theory various drastically with the method employed.

Acknowledgements • Dr. Kenneth Leopold • Jane Curtis • Dr. Carolyn Brauer Funding • National Science Foundation (NSF) • Petroleum Research Fund (PRF) • Minnesota Supercomputing Institute (MSI)

 Sulfuric acid dipole moment
Sulfuric acid dipole moment Dipole dipole vs ion dipole
Dipole dipole vs ion dipole Is sulfuric acid a weak acid
Is sulfuric acid a weak acid Dipole dipole interaction
Dipole dipole interaction Ion induced dipole examples
Ion induced dipole examples Strength of intermolecular forces
Strength of intermolecular forces Dispersion forces vs dipole dipole
Dispersion forces vs dipole dipole Dipole dipole bond
Dipole dipole bond Force de london
Force de london Capillary action
Capillary action Dipole dipole interaction example
Dipole dipole interaction example Dispersion forces vs dipole dipole
Dispersion forces vs dipole dipole Instantaneous dipole moment
Instantaneous dipole moment Dipole dipole interaction
Dipole dipole interaction Intermolecular forces formaldehyde
Intermolecular forces formaldehyde Dipole-dipole attractions
Dipole-dipole attractions Hidroxid de sodiu + acid sulfuric
Hidroxid de sodiu + acid sulfuric Urea sulfuric acid
Urea sulfuric acid S22fe
S22fe Sulfuric acid mist
Sulfuric acid mist Concentrated sulfuric acid electrolysis
Concentrated sulfuric acid electrolysis Contact process
Contact process Can more things be dissolved in sulfuric acid than water
Can more things be dissolved in sulfuric acid than water Physical hazards
Physical hazards