Terapia Neoadiuvante Revisione delle evidenze scientifiche Valentina Guarneri






- Slides: 27

Terapia Neoadiuvante Revisione delle evidenze scientifiche Valentina Guarneri Nonantola, 19 Novembre 2011

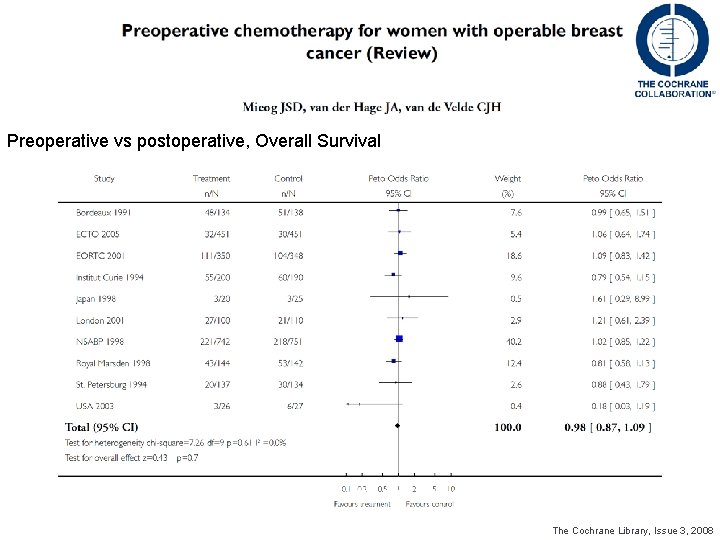
Preoperative vs postoperative, Overall Survival DDFS o OS? ? ? The Cochrane Library, Issue 3, 2008

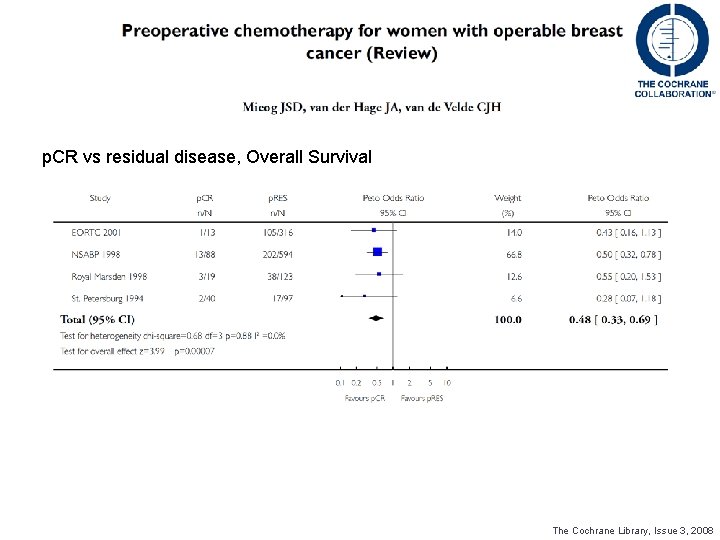
p. CR vs residual disease, Overall Survival The Cochrane Library, Issue 3, 2008

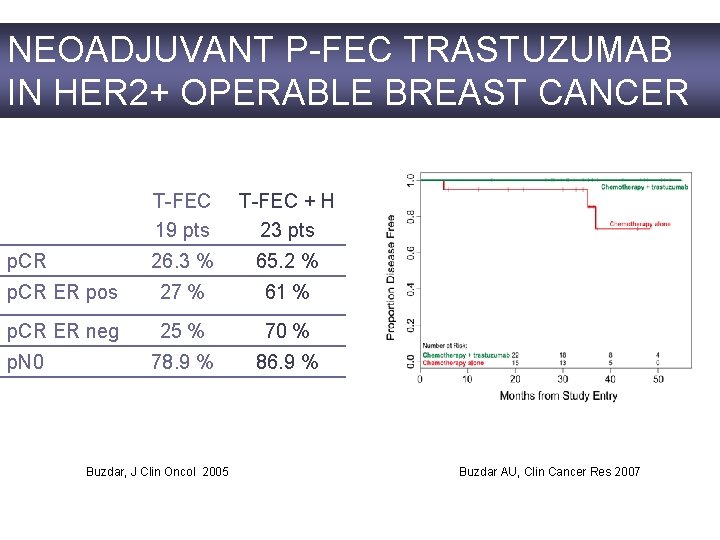
NEOADJUVANT P-FEC TRASTUZUMAB IN HER 2+ OPERABLE BREAST CANCER T-FEC 19 pts T-FEC + H 23 pts 26. 3 % 65. 2 % p. CR ER pos 27 % 61 % p. CR ER neg 25 % 70 % 78. 9 % 86. 9 % p. CR p. N 0 Buzdar, J Clin Oncol 2005 Buzdar AU, Clin Cancer Res 2007

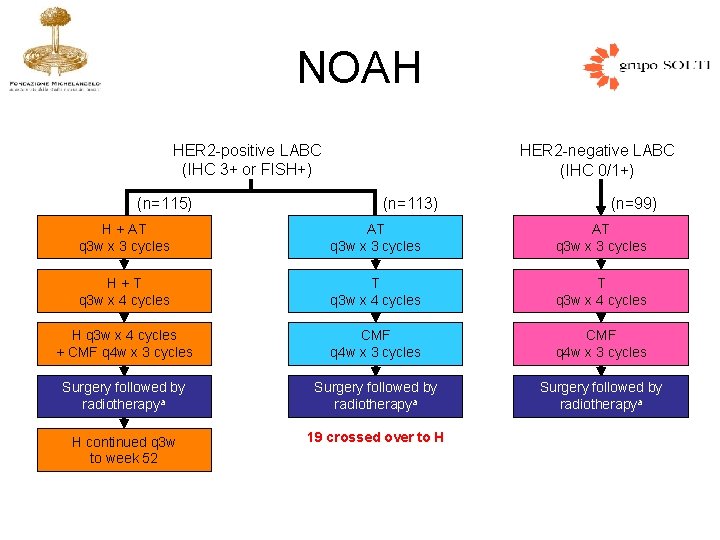
NOAH HER 2 -positive LABC (IHC 3+ or FISH+) (n=115) HER 2 -negative LABC (IHC 0/1+) (n=113) (n=99) H + AT q 3 w x 3 cycles H+T q 3 w x 4 cycles H q 3 w x 4 cycles + CMF q 4 w x 3 cycles Surgery followed by radiotherapya H continued q 3 w to week 52 19 crossed over to H

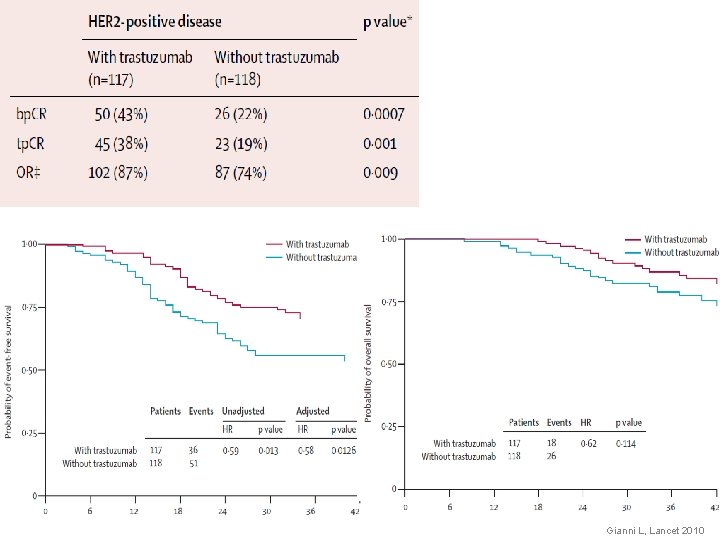
Gianni L, Lancet 2010

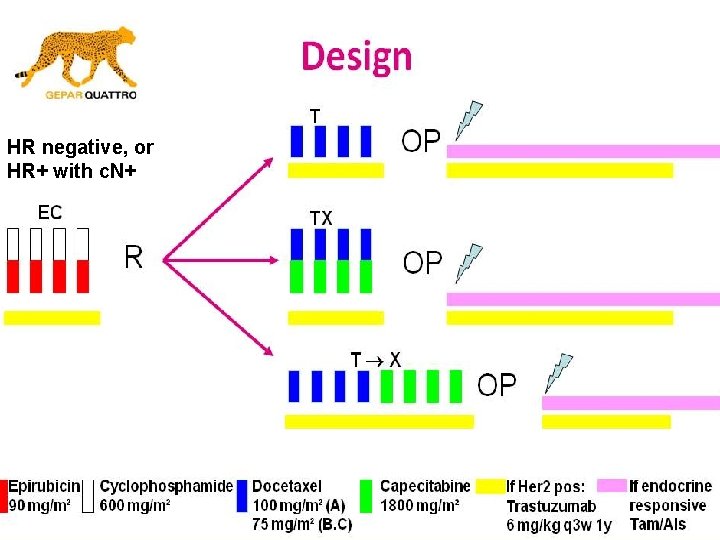
HR negative, or HR+ with c. N+

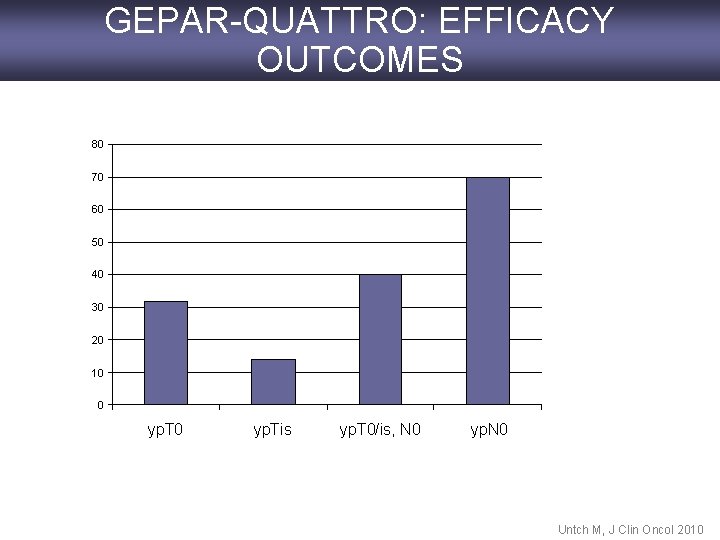
GEPAR-QUATTRO: EFFICACY OUTCOMES 80 70 60 50 40 30 20 10 0 yp. Tis yp. T 0/is, N 0 yp. N 0 Untch M, J Clin Oncol 2010

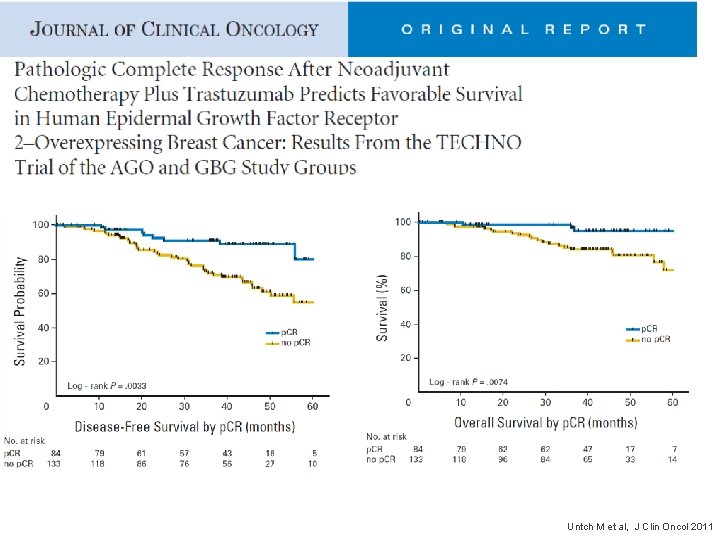
Untch M et al, J Clin Oncol 2011





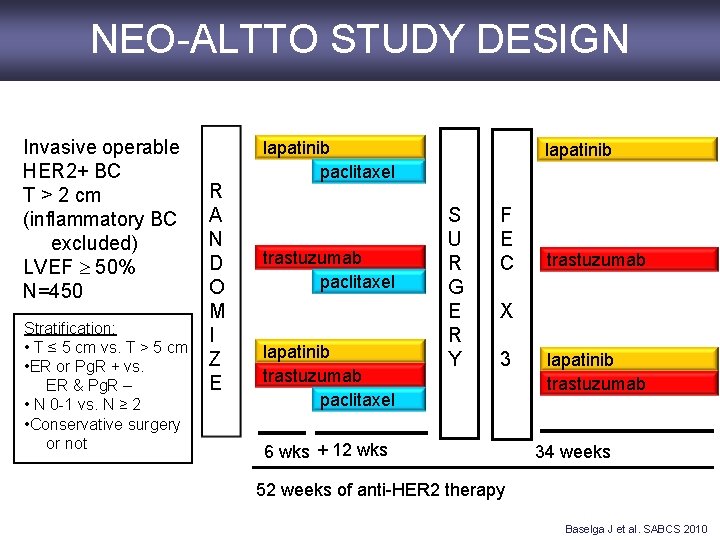
NEO-ALTTO STUDY DESIGN Invasive operable HER 2+ BC T > 2 cm (inflammatory BC excluded) LVEF 50% N=450 Stratification: • T ≤ 5 cm vs. T > 5 cm • ER or Pg. R + vs. ER & Pg. R – • N 0 -1 vs. N ≥ 2 • Conservative surgery or not R A N D O M I Z E lapatinib paclitaxel trastuzumab paclitaxel lapatinib S U R G E R Y F E C trastuzumab X 3 6 wks + 12 wks lapatinib trastuzumab 34 weeks 52 weeks of anti-HER 2 therapy Baselga J et al. SABCS 2010

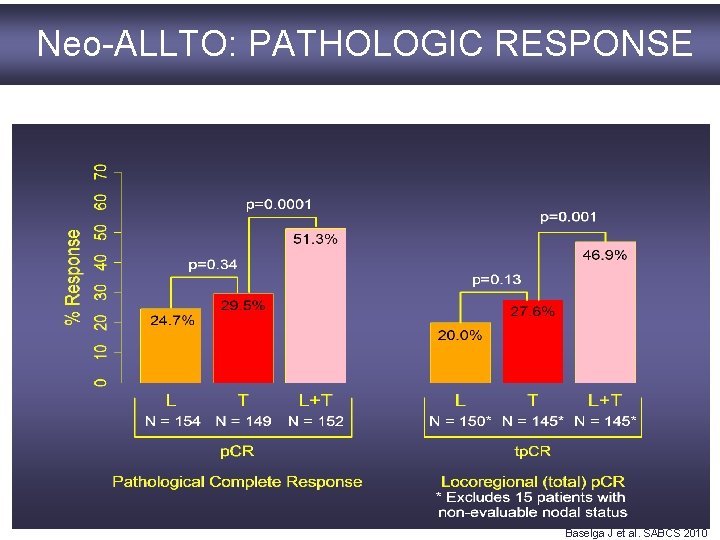
Neo-ALLTO: PATHOLOGIC RESPONSE L: lapatinib; T: trastuzumab; L+T: lapatinib plus trastuzumab p. CR pathologic complete response Baselga J et al. SABCS 2010

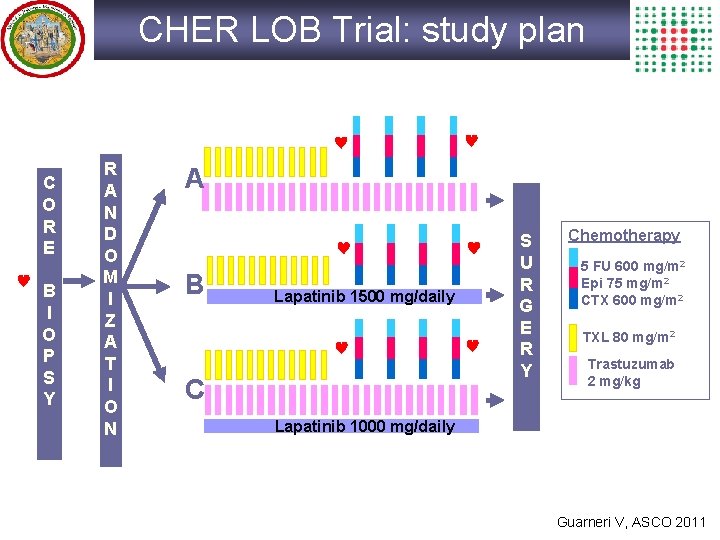
CHER LOB Trial: study plan C O R E B I O P S Y R A N D O M I Z A T I O N A B Lapatinib 1500 mg/daily C S U R G E R Y Chemotherapy 5 FU 600 mg/m 2 Epi 75 mg/m 2 CTX 600 mg/m 2 TXL 80 mg/m 2 Trastuzumab 2 mg/kg Lapatinib 1000 mg/daily Guarneri V, ASCO 2011

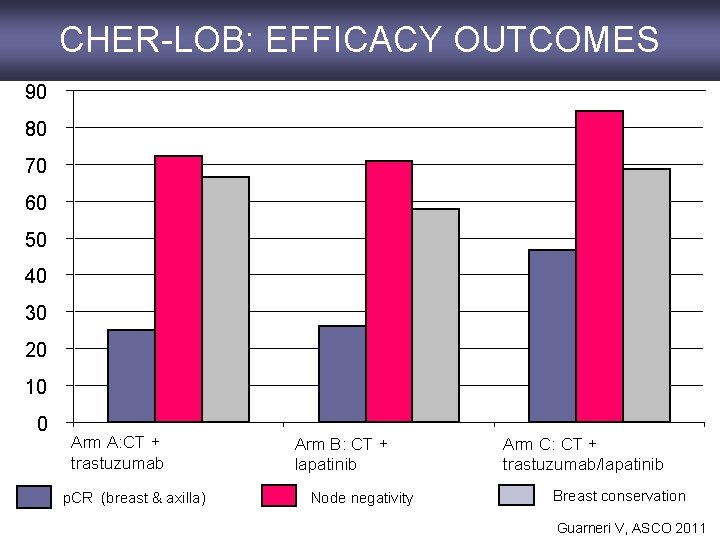
CHER-LOB: EFFICACY OUTCOMES 90 80 70 60 50 40 30 20 10 0 Arm A: CT + trastuzumab p. CR (breast & axilla) Arm B: CT + lapatinib Node negativity Arm C: CT + trastuzumab/lapatinib Breast conservation Guarneri V, ASCO 2011

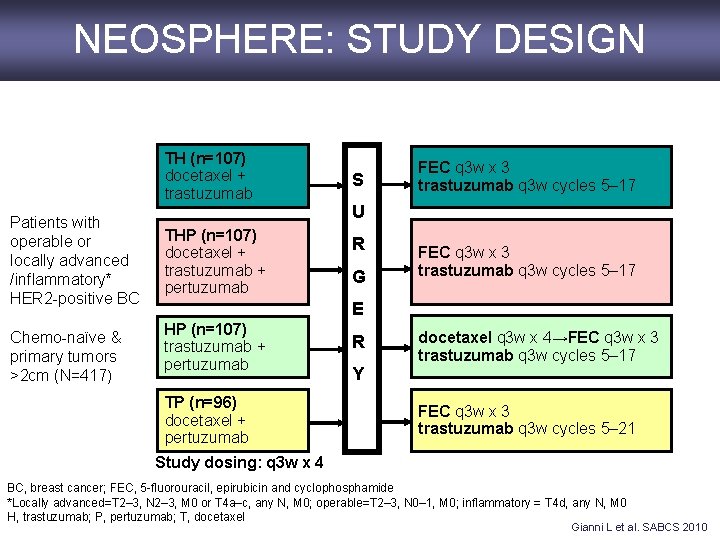
NEOSPHERE: STUDY DESIGN TH (n=107) docetaxel + trastuzumab Patients with operable or locally advanced /inflammatory* HER 2 -positive BC Chemo-naïve & primary tumors >2 cm (N=417) THP (n=107) docetaxel + trastuzumab + pertuzumab S FEC q 3 w x 3 trastuzumab q 3 w cycles 5– 17 U R G FEC q 3 w x 3 trastuzumab q 3 w cycles 5– 17 E HP (n=107) trastuzumab + pertuzumab TP (n=96) docetaxel + pertuzumab R Y docetaxel q 3 w x 4→FEC q 3 w x 3 trastuzumab q 3 w cycles 5– 17 FEC q 3 w x 3 trastuzumab q 3 w cycles 5– 21 Study dosing: q 3 w x 4 BC, breast cancer; FEC, 5 -fluorouracil, epirubicin and cyclophosphamide *Locally advanced=T 2– 3, N 2– 3, M 0 or T 4 a–c, any N, M 0; operable=T 2– 3, N 0– 1, M 0; inflammatory = T 4 d, any N, M 0 H, trastuzumab; P, pertuzumab; T, docetaxel Gianni L et al. SABCS 2010

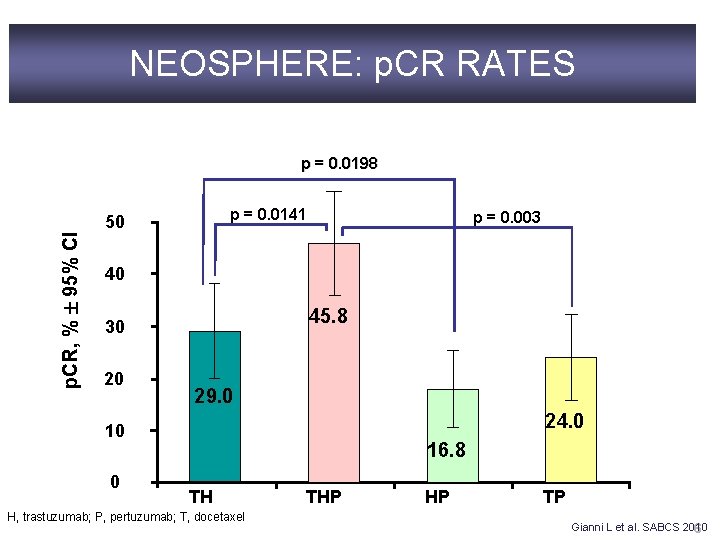
NEOSPHERE: p. CR RATES p. CR, % 95% CI p = 0. 0198 p = 0. 0141 50 p = 0. 003 40 45. 8 30 20 29. 0 24. 0 10 0 16. 8 TH H, trastuzumab; P, pertuzumab; T, docetaxel THP HP TP Gianni L et al. SABCS 2010 6

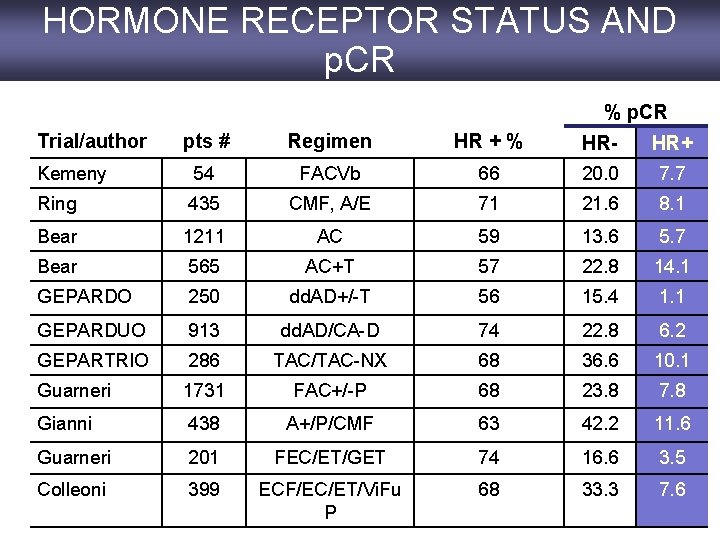
HORMONE RECEPTOR STATUS AND p. CR % p. CR Trial/author pts # Regimen HR + % HR- HR+ Kemeny 54 FACVb 66 20. 0 7. 7 Ring 435 CMF, A/E 71 21. 6 8. 1 Bear 1211 AC 59 13. 6 5. 7 Bear 565 AC+T 57 22. 8 14. 1 GEPARDO 250 dd. AD+/-T 56 15. 4 1. 1 GEPARDUO 913 dd. AD/CA-D 74 22. 8 6. 2 GEPARTRIO 286 TAC/TAC-NX 68 36. 6 10. 1 Guarneri 1731 FAC+/-P 68 23. 8 7. 8 Gianni 438 A+/P/CMF 63 42. 2 11. 6 Guarneri 201 FEC/ET/GET 74 16. 6 3. 5 Colleoni 399 ECF/EC/ET/Vi. Fu P 68 33. 3 7. 6

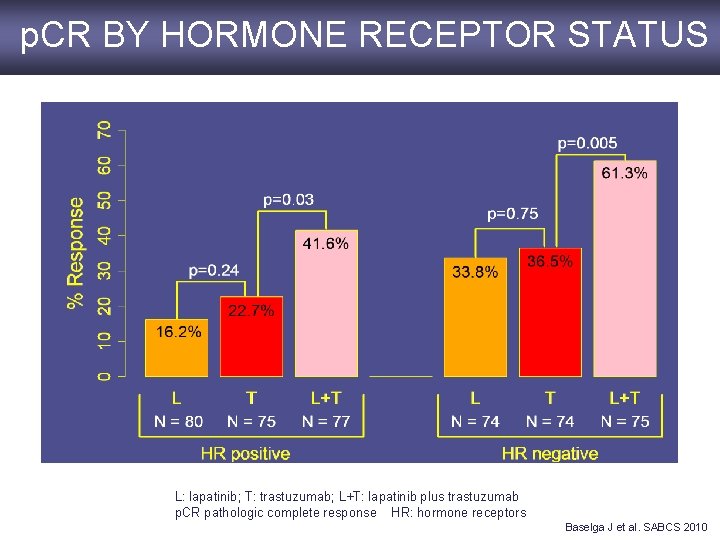
p. CR BY HORMONE RECEPTOR STATUS L: lapatinib; T: trastuzumab; L+T: lapatinib plus trastuzumab p. CR pathologic complete response HR: hormone receptors Baselga J et al. SABCS 2010

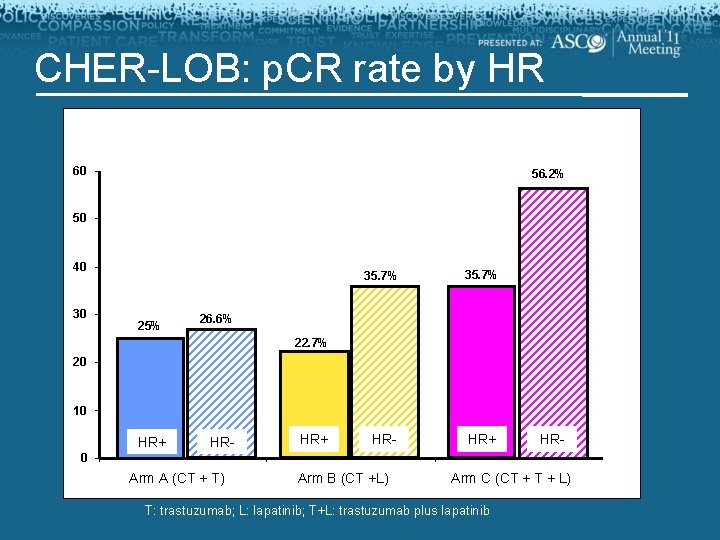
CHER-LOB: p. CR rate by HR 60 56. 2% 50 40 30 25% 35. 7% HR- HR+ 26. 6% 22. 7% 20 10 HR+ HR- 0 Arm A (CT + T) Arm B (CT +L) Arm C (CT + L) T: trastuzumab; L: lapatinib; T+L: trastuzumab plus lapatinib

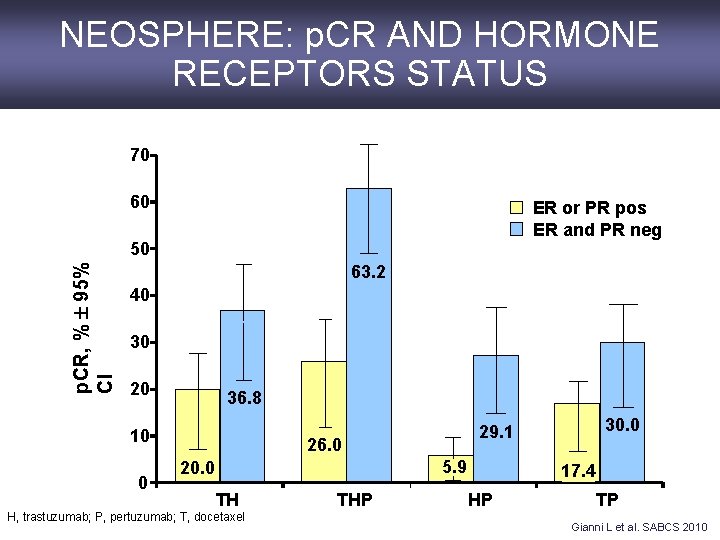
NEOSPHERE: p. CR AND HORMONE RECEPTORS STATUS 70 60 ER or PR pos ER and PR neg p. CR, % 95% CI 50 63. 2 40 30 20 36. 8 10 0 26. 0 5. 9 20. 0 TH H, trastuzumab; P, pertuzumab; T, docetaxel THP 30. 0 29. 1 17. 4 HP TP Gianni L et al. SABCS 2010

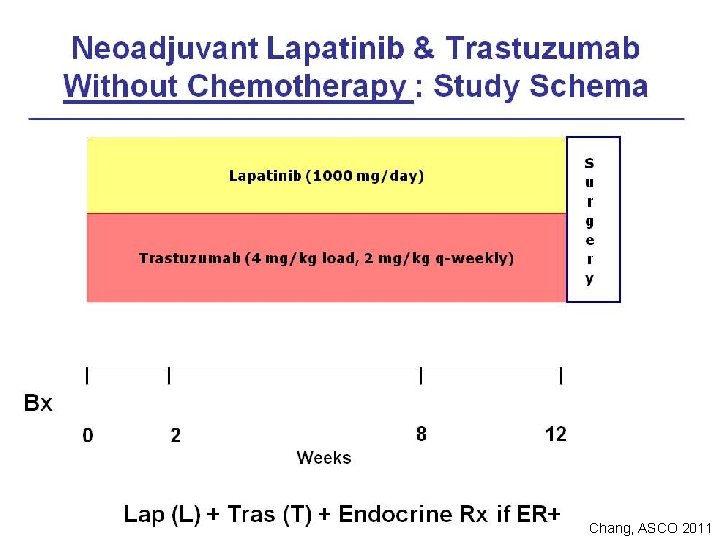
Chang, ASCO 2011

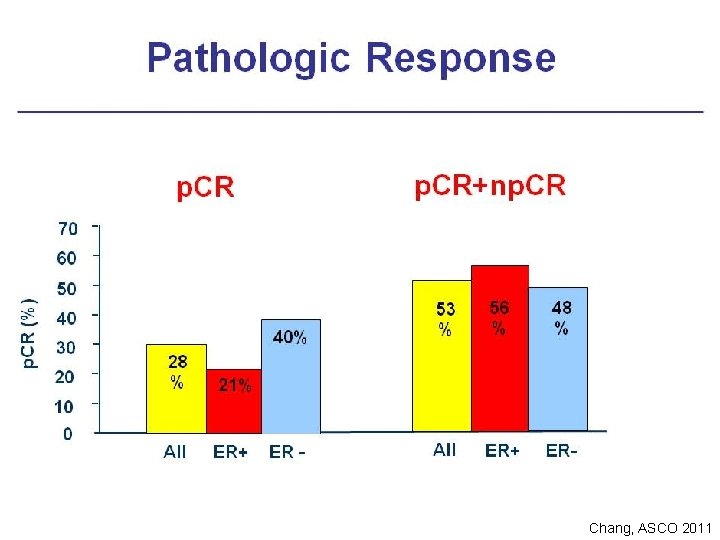
Chang, ASCO 2011

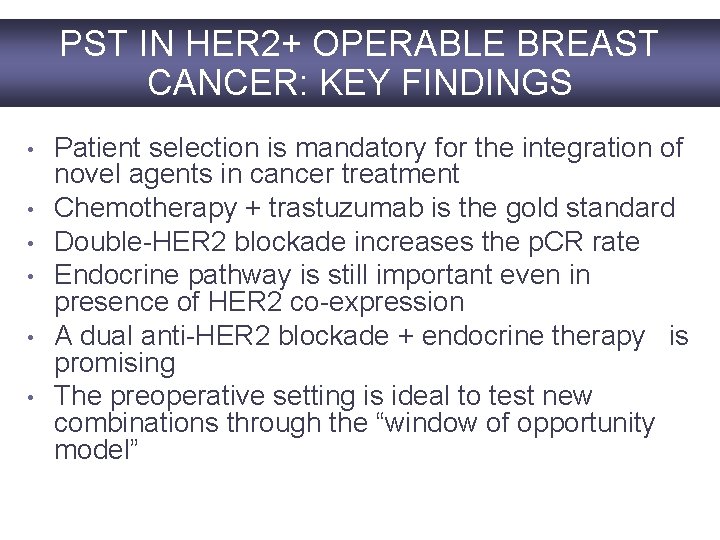
PST IN HER 2+ OPERABLE BREAST CANCER: KEY FINDINGS • • • Patient selection is mandatory for the integration of novel agents in cancer treatment Chemotherapy + trastuzumab is the gold standard Double-HER 2 blockade increases the p. CR rate Endocrine pathway is still important even in presence of HER 2 co-expression A dual anti-HER 2 blockade + endocrine therapy is promising The preoperative setting is ideal to test new combinations through the “window of opportunity model”

 Piramide evidenze scientifiche
Piramide evidenze scientifiche Valutazione comportamento scuola media
Valutazione comportamento scuola media Lettera di attestazione
Lettera di attestazione Relazione stato avanzamento lavori modello doc
Relazione stato avanzamento lavori modello doc Revisione tra pari
Revisione tra pari Sicob sleeve gastrectomy
Sicob sleeve gastrectomy Divisione con notazione scientifica
Divisione con notazione scientifica Scoperte scientifiche durante il romanticismo
Scoperte scientifiche durante il romanticismo Belle epoque scoperte scientifiche
Belle epoque scoperte scientifiche Scoperte scientifiche dal 1850 al 1900
Scoperte scientifiche dal 1850 al 1900 La nascita delle lingue e delle letterature romanze
La nascita delle lingue e delle letterature romanze L esperienza delle cose moderne e la lezione delle antique
L esperienza delle cose moderne e la lezione delle antique Lezione delle antique cose
Lezione delle antique cose Valentina draksler
Valentina draksler Ivan milat
Ivan milat Colors that are opposite to each other
Colors that are opposite to each other Valentina cantillana
Valentina cantillana Maslow
Maslow Valentina pineda xxx
Valentina pineda xxx Pandektno pravo
Pandektno pravo Comixtio
Comixtio Valentina cattivelli
Valentina cattivelli Valentina felici
Valentina felici Valentina boskovic markovic
Valentina boskovic markovic Rebecca valentina
Rebecca valentina Fotonica
Fotonica Valentina giorgetti
Valentina giorgetti Valentina ricciardi
Valentina ricciardi