SWORD 1 2 Switch to DTG RPV Maintains

- Slides: 16

SWORD 1 & 2: Switch to DTG + RPV Maintains Virologic Suppression Through 48 Weeks, a Phase III Study JM Llibre, 1 C-C Hung, 2 C Brinson, 3 F Castelli, 4 P-M Girard, 5 LP Kahl, 6 E Blair, 7 B Wynne, 8 K Vandermeulen, 9 M Aboud 10 1 Univ Hosp Germans Trias, Badalona, Barcelona; 2 National Taiwan University Hospital, Taipei, Taiwan; 3 Central Texas Clinical Research, Austin, TX; 4 University Department of Infectious and Tropical Diseases, ASST Spedali Civili di Brescia, Italy; 5 Saint-Antoine Hospital, AP-HP, Paris, France; 6 Glaxo. Smith. Kline, Uxbridge, United Kingdom; 7 Vii. V Healthcare, Research Triangle Park, NC; 8 Vii. V Healthcare, Collegeville, PA; 9 Janssen, Beerse, Belgium; 10 Vii. V Healthcare, Brentford, United Kingdom Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

Introduction • The requirement for life-long antiretroviral therapy (ART) for HIV infection has highlighted a need to minimize cumulative drug exposure • The potency, safety, and resistance barrier of dolutegravir (DTG) make it an ideal core agent for two-drug regimen (2 DR) • The safety, tolerability, and efficacy of rilpivirine (RPV) make it an optimal partner • The SWORD-1&2 studies evaluated whether a 2 DR of DTG + RPV once daily was as effective as a 3 - or 4 DR for the maintenance of virologic suppression 1. Raffi et al. HIV Med. 2016; 17(suppl 5): 3 -16. 2. Ford et al. Antimicrob Agents Chemother. 2013; 57: 5472 -5477. 3. Palella et al. AIDS. 2014; 28: 335 -344. Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

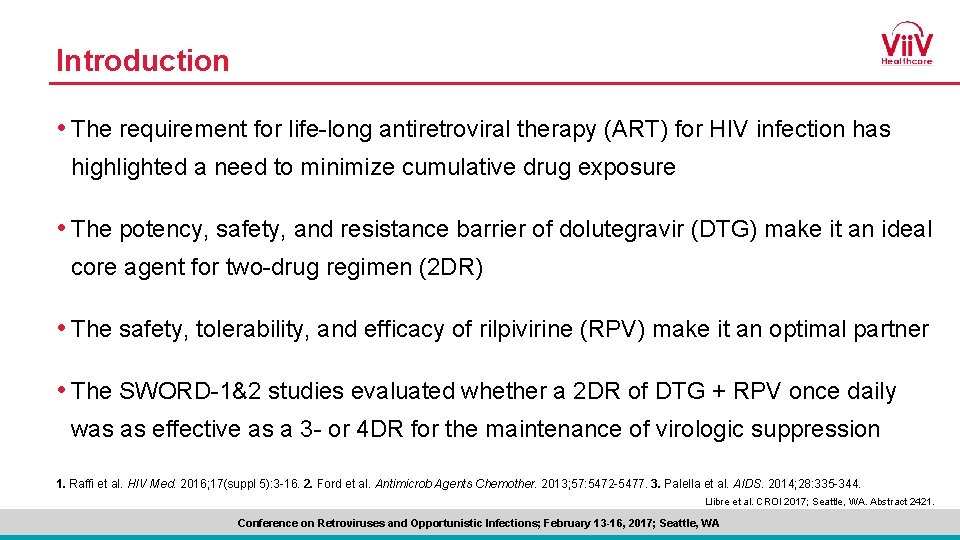
SWORD-1 and SWORD-2 Phase III Study Design Identically designed, randomized, multicenter, open-label, parallel-group, non-inferiority studies Screening Early switch phase 1: 1 Continuation phase DTG + RPV (N=513) VL <50 c/m. L on INI, NNRTI, or PI + 2 NRTIs Inclusion criteria • On stable CAR >6 months before screening • 1 st or 2 nd ART with no change in prior regimen due to VF • Confirmed HIV-1 RNA <50 c/m. L during the 12 months before screening • HBV negative DTG + RPV CAR (N=511) Day 1 a-8% Late switch phase Week 52 Primary endpoint at 48 weeks: subjects with VL <50 c/m. L (ITT-E snapshot)a Week 148 Countries Argentina Australia France Germany Russia Spain United States Belgium Italy Taiwan Canada Netherlands United Kingdom non-inferiority margin for pooled data. -10% non-inferiority margin for individual studies Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

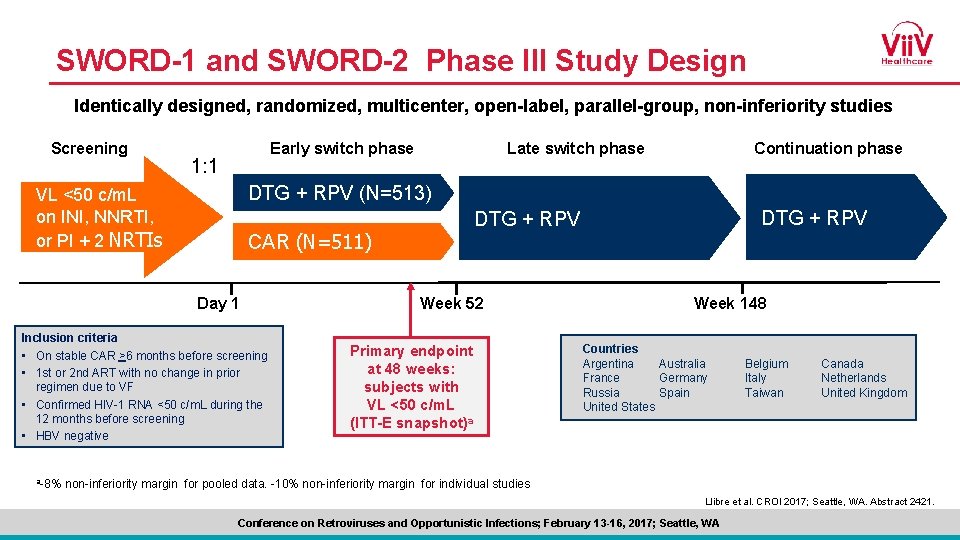
Subject Disposition: Early Switch Phase (Through Wk 52) Screeneda N=1339 Randomized and treated DTG + RPV n=513 Randomized and treated CAR n=511 Contributed VL to Wk 48 n=486 (95%) Completed Early Switch Phaseb n=484 (94%) Contributed VL to Wk 48 n=487 (95%) Withdrawals through Wk 52 n=29 (6%) Adverse event Investigator discretion a. Data 17 (3%) 0 Completed Early Switch Phaseb n=477 (93%) Withdrawals through Wk 52 n=34 (7%) Adverse event 3 (<1%) Investigator discretion 3 (<1%) Lack of efficacy 3 (<1%) Lost to follow-up 2 (<1%) Lost to follow-up 3 (<1%) Protocol deviation 1 (<1%) Protocol deviation 7 (1%) Protocol-defined stopping criteria 1 (<1%) Withdrew consent 5 (<1%) Withdrew consent 14 (3%) pooled across SWORD-1 and SWORD-2. b. Early switch phase ends at Week 52. Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

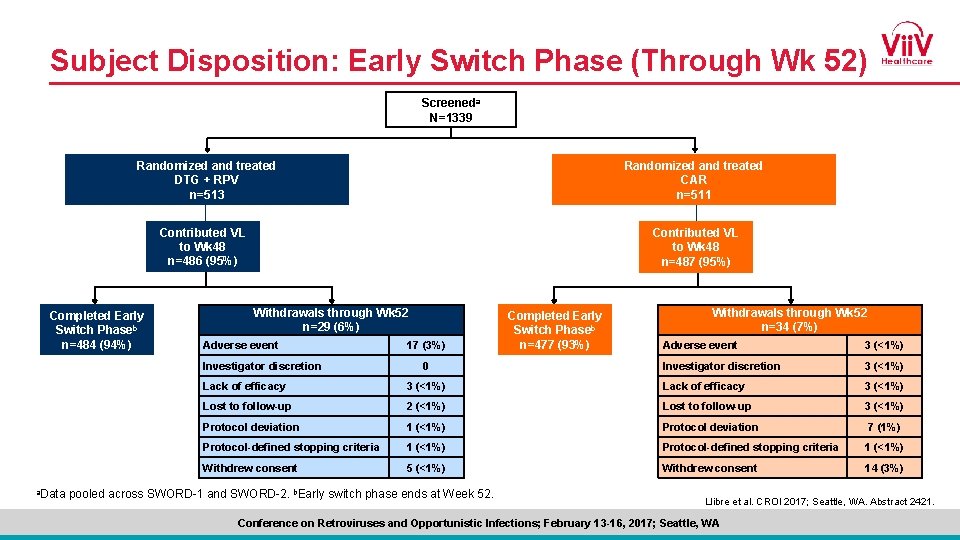
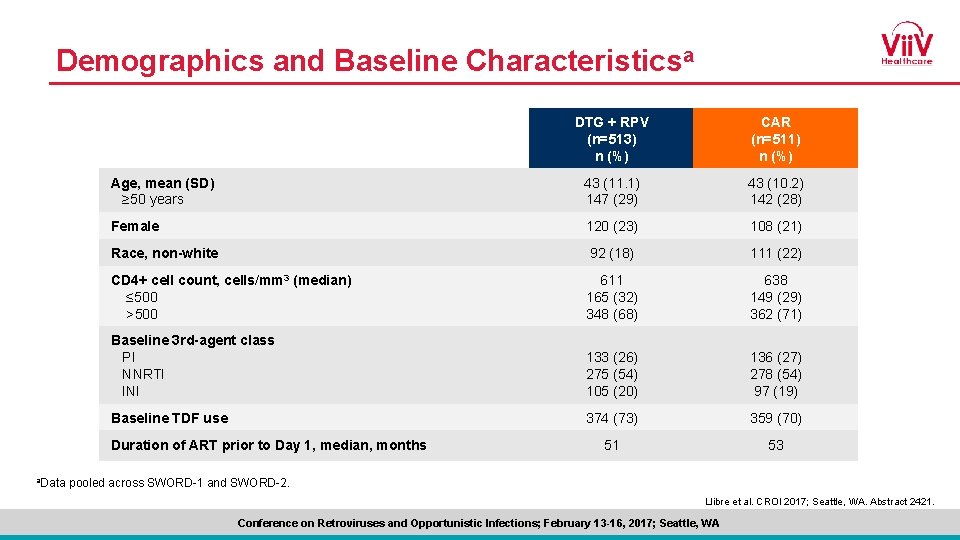
Demographics and Baseline Characteristicsa DTG + RPV (n=513) n (%) CAR (n=511) n (%) Age, mean (SD) ≥ 50 years 43 (11. 1) 147 (29) 43 (10. 2) 142 (28) Female 120 (23) 108 (21) Race, non-white 92 (18) 111 (22) CD 4+ cell count, cells/mm 3 (median) ≤ 500 >500 611 165 (32) 348 (68) 638 149 (29) 362 (71) Baseline 3 rd-agent class PI NNRTI INI 133 (26) 275 (54) 105 (20) 136 (27) 278 (54) 97 (19) Baseline TDF use 374 (73) 359 (70) 51 53 Duration of ART prior to Day 1, median, months a. Data pooled across SWORD-1 and SWORD-2. Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

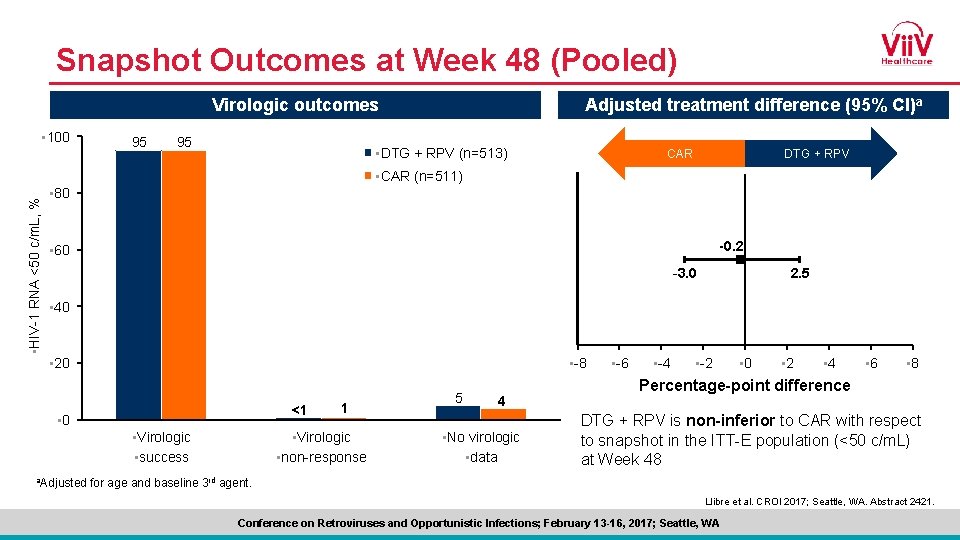
Snapshot Outcomes at Week 48 (Pooled) Virologic outcomes • 100 95 95 Adjusted treatment difference (95% CI)a • DTG + RPV (n=513) DTG + RPV CAR • HIV-1 RNA <50 c/m. L, % • CAR (n=511) • 80 -0. 2 • 60 -3. 0 2. 5 • 40 • -8 • 20 <1 • 0 • Virologic • success a. Adjusted 1 • Virologic • non-response 5 4 • No virologic • data • -6 • -4 • -2 • 0 • 2 • 4 • 6 • 8 Percentage-point difference DTG + RPV is non-inferior to CAR with respect to snapshot in the ITT-E population (<50 c/m. L) at Week 48 for age and baseline 3 rd agent. Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

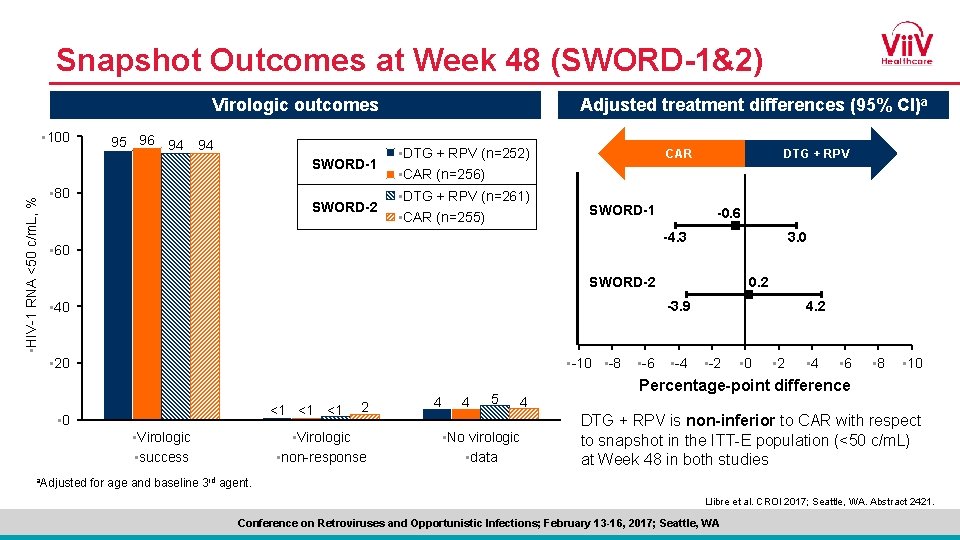
Snapshot Outcomes at Week 48 (SWORD-1&2) Virologic outcomes • 100 95 96 94 94 • HIV-1 RNA <50 c/m. L, % SWORD-1 • 80 SWORD-2 Adjusted treatment differences (95% CI)a • DTG + RPV (n=252) • CAR (n=256) • DTG + RPV (n=261) • CAR (n=255) DTG + RPV CAR SWORD-1 -0. 6 -4. 3 • 60 3. 0 0. 2 SWORD-2 -3. 9 • 40 • -10 • -8 • 20 <1 <1 <1 • 0 • Virologic • success a. Adjusted 2 • Virologic • non-response 4 4 5 4 • No virologic • data • -6 • -4 4. 2 • -2 • 0 • 2 • 4 • 6 • 8 • 10 Percentage-point difference DTG + RPV is non-inferior to CAR with respect to snapshot in the ITT-E population (<50 c/m. L) at Week 48 in both studies for age and baseline 3 rd agent. Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

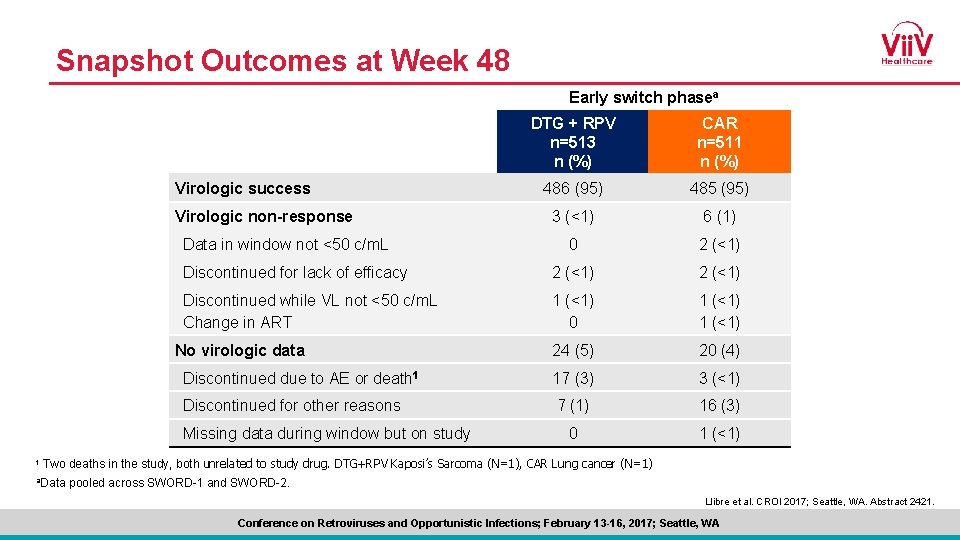
Snapshot Outcomes at Week 48 Early switch phasea DTG + RPV n=513 n (%) CAR n=511 n (%) 486 (95) 485 (95) 3 (<1) 6 (1) 0 2 (<1) Discontinued for lack of efficacy 2 (<1) Discontinued while VL not <50 c/m. L Change in ART 1 (<1) 0 1 (<1) 24 (5) 20 (4) Discontinued due to AE or death 1 17 (3) 3 (<1) Discontinued for other reasons 7 (1) 16 (3) 0 1 (<1) Virologic success Virologic non-response Data in window not <50 c/m. L No virologic data Missing data during window but on study 1 Two deaths in the study, both unrelated to study drug. DTG+RPV Kaposi’s Sarcoma (N=1), CAR Lung cancer (N=1) a. Data pooled across SWORD-1 and SWORD-2. Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

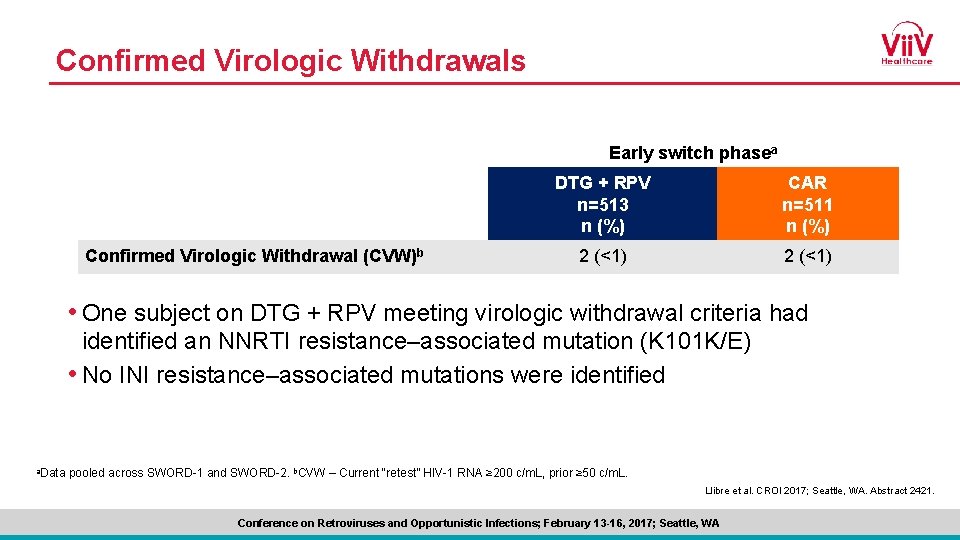
Confirmed Virologic Withdrawals Early switch phasea Confirmed Virologic Withdrawal (CVW)b DTG + RPV n=513 n (%) CAR n=511 n (%) 2 (<1) • One subject on DTG + RPV meeting virologic withdrawal criteria had identified an NNRTI resistance–associated mutation (K 101 K/E) • No INI resistance–associated mutations were identified a. Data pooled across SWORD-1 and SWORD-2. b. CVW – Current “retest” HIV-1 RNA ≥ 200 c/m. L, prior ≥ 50 c/m. L. Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

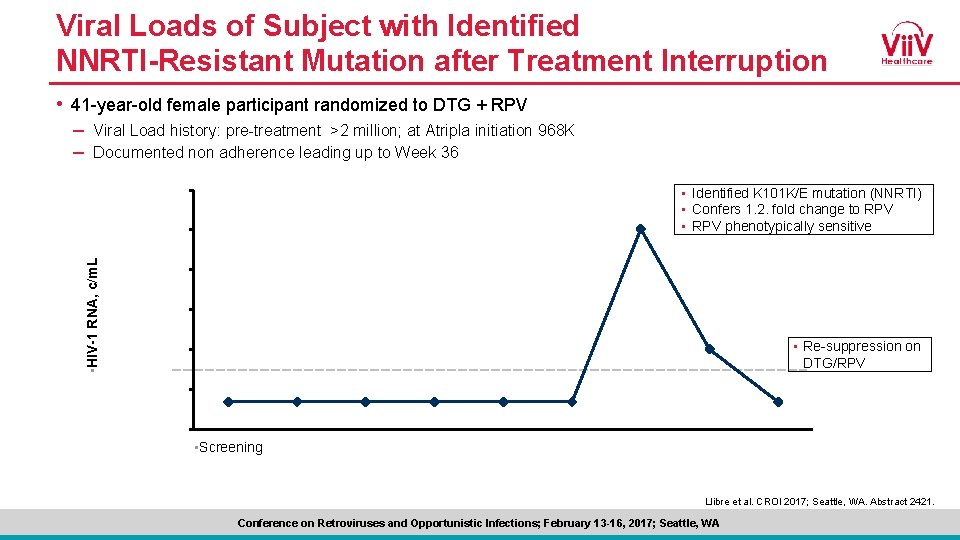
Viral Loads of Subject with Identified NNRTI-Resistant Mutation after Treatment Interruption • 41 -year-old female participant randomized to DTG + RPV – Viral Load history: pre-treatment >2 million; at Atripla initiation 968 K – Documented non adherence leading up to Week 36 • HIV-1 RNA, c/m. L • Identified K 101 K/E mutation (NNRTI) • Confers 1. 2. fold change to RPV • RPV phenotypically sensitive • Re-suppression on DTG/RPV • Screening Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

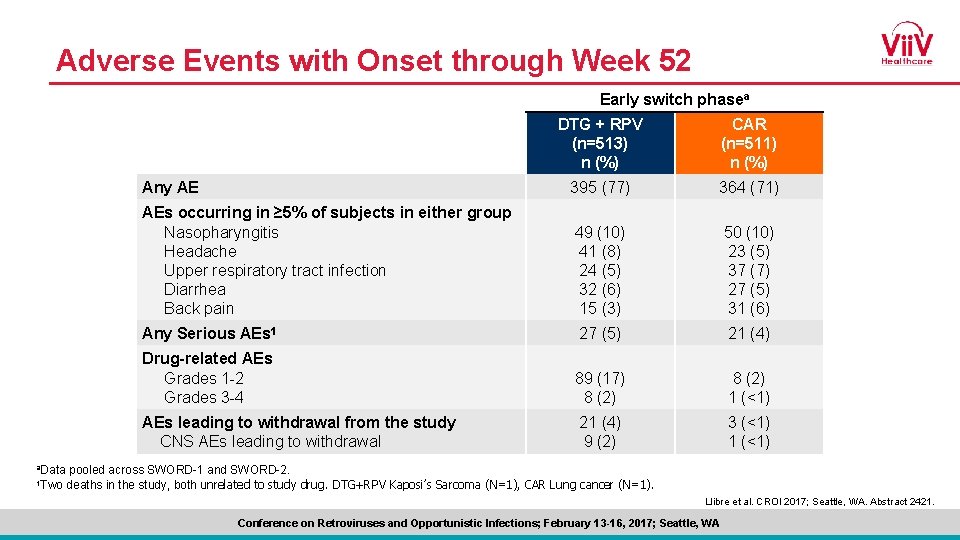
Adverse Events with Onset through Week 52 Early switch phasea a. Data 1 Two DTG + RPV (n=513) n (%) CAR (n=511) n (%) Any AE 395 (77) 364 (71) AEs occurring in ≥ 5% of subjects in either group Nasopharyngitis Headache Upper respiratory tract infection Diarrhea Back pain 49 (10) 41 (8) 24 (5) 32 (6) 15 (3) 50 (10) 23 (5) 37 (7) 27 (5) 31 (6) Any Serious AEs 1 27 (5) 21 (4) Drug-related AEs Grades 1 -2 Grades 3 -4 89 (17) 8 (2) 1 (<1) AEs leading to withdrawal from the study CNS AEs leading to withdrawal 21 (4) 9 (2) 3 (<1) 1 (<1) pooled across SWORD-1 and SWORD-2. deaths in the study, both unrelated to study drug. DTG+RPV Kaposi’s Sarcoma (N=1), CAR Lung cancer (N=1). Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

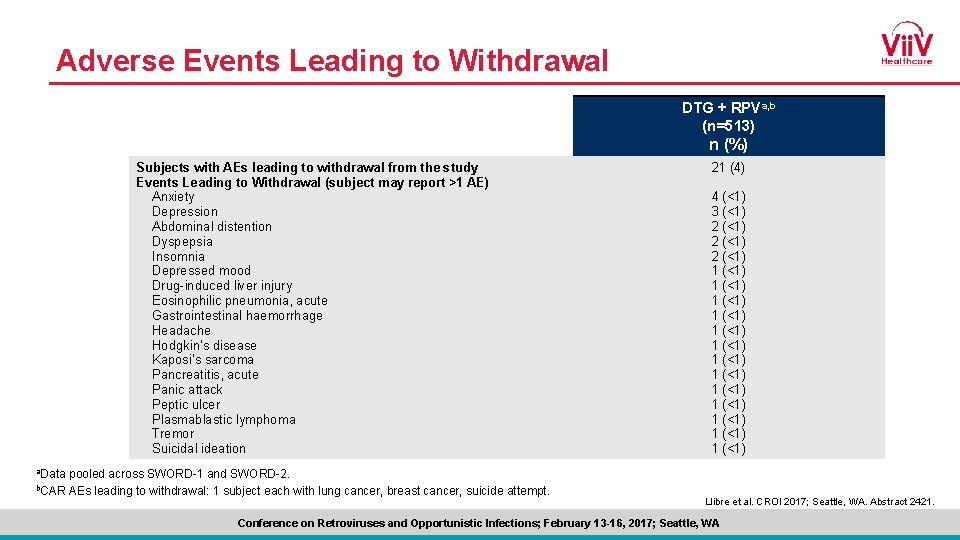
Adverse Events Leading to Withdrawal DTG + RPVa, b (n=513) n (%) Subjects with AEs leading to withdrawal from the study Events Leading to Withdrawal (subject may report >1 AE) Anxiety Depression Abdominal distention Dyspepsia Insomnia Depressed mood Drug-induced liver injury Eosinophilic pneumonia, acute Gastrointestinal haemorrhage Headache Hodgkin’s disease Kaposi’s sarcoma Pancreatitis, acute Panic attack Peptic ulcer Plasmablastic lymphoma Tremor Suicidal ideation a. Data b. CAR pooled across SWORD-1 and SWORD-2. AEs leading to withdrawal: 1 subject each with lung cancer, breast cancer, suicide attempt. 21 (4) 4 (<1) 3 (<1) 2 (<1) 1 (<1) 1 (<1) 1 (<1) 1 (<1) Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

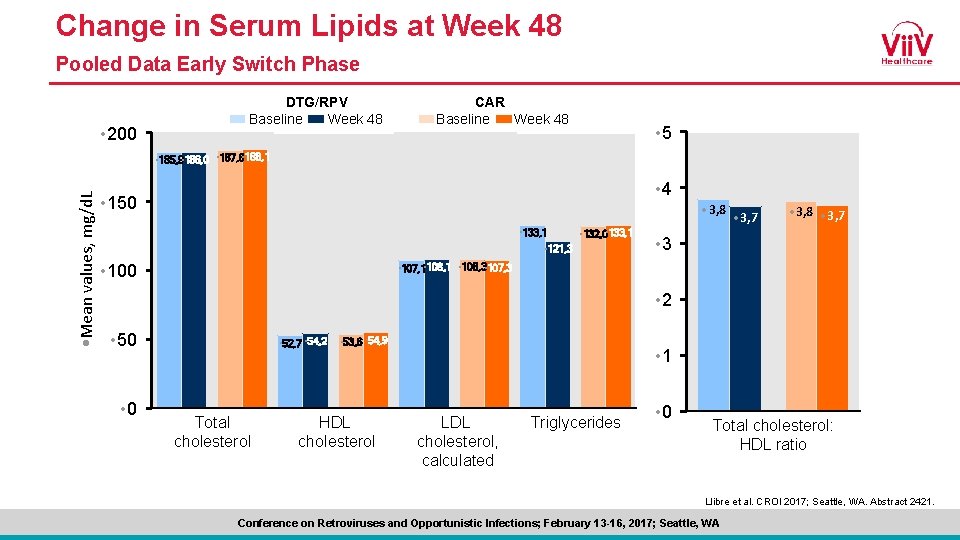
Change in Serum Lipids at Week 48 Pooled Data Early Switch Phase • 200 DTG/RPV Baseline Week 48 CAR Baseline Week 48 • 5 • Mean values, mg/d. L • 185, 9 • 186, 0 • 187, 6 • 188, 1 • 4 • 150 • 3, 8 • 3, 7 • 133, 1 • 132, 0 • 133, 1 • 121, 3 • 100 • 3, 8 • 3, 7 • 3 • 107, 1 • 108, 3 • 107, 3 • 2 • 50 • 52, 7 • 54, 2 Total cholesterol • 53, 6 • 54, 9 HDL cholesterol • 1 LDL cholesterol, calculated Triglycerides • 0 Total cholesterol: HDL ratio Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

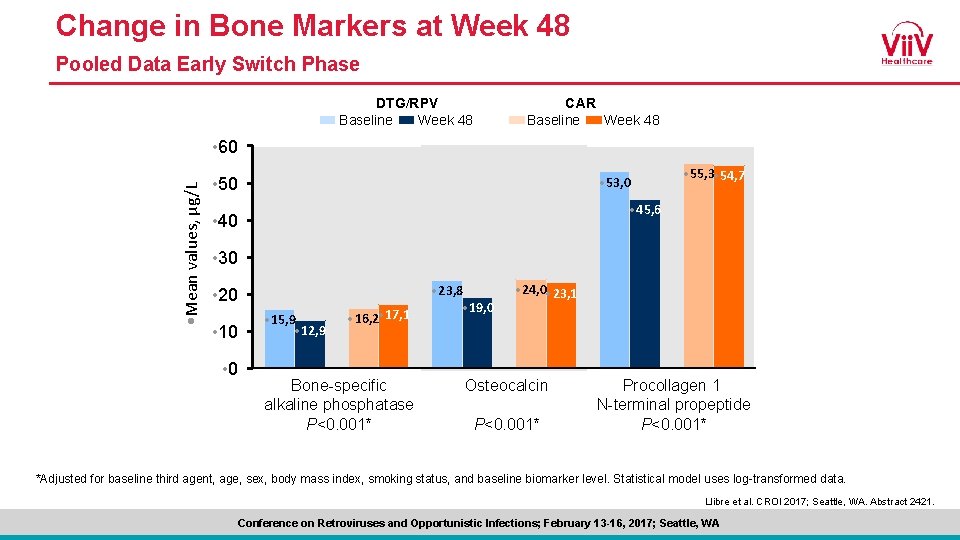
Change in Bone Markers at Week 48 Pooled Data Early Switch Phase CAR Baseline Week 48 DTG/RPV Baseline Week 48 • Mean values, µg/L • 60 • 53, 0 • 55, 3 • 54, 7 • 45, 6 • 40 • 30 • 20 • 10 • 0 • 15, 9 • 12, 9 • 16, 2 • 17, 1 Bone-specific alkaline phosphatase P<0. 001* • 23, 8 • 19, 0 • 24, 0 • 23, 1 Osteocalcin P<0. 001* Procollagen 1 N-terminal propeptide P<0. 001* *Adjusted for baseline third agent, age, sex, body mass index, smoking status, and baseline biomarker level. Statistical model uses log-transformed data. Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

Conclusions • A switch to a novel, once-daily 2 DR of DTG + RPV demonstrated high efficacy and was non-inferior to the continuation of a 3 - or 4 DR in virologically suppressed HIV-1–infected adults • The safety profiles of both DTG and RPV were consistent with their respective labels • Switching to DTG+RPV had a neutral effect on lipids, while significantly improving bone turnover biomarkers • These data support the use of DTG+RPV as a 2 DR for streamlining therapy for maintenance of suppression • These data support – Regulatory filing for DTG/RPV – Exploration of additional regimens in the 2 DR paradigm Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA

Acknowledgments • We thank everyone who has contributed to the success of these studies, including – All study participants and their families – The SWORD-1 and SWORD-2 clinical investigators and their staff Argentina Cahn Cassetti Lopardo Lupo Australia Baker Bloch Finlayson Roth Smith Belgium De Wit Florence Moutschen Vandekerckhove Canada Angel Charest Conway Cote De Wet Fraser Leblanc Rachlis Routy Tan Trottier Walmsley Wong France Ajana Cotte De Truchis Girard Katlama Khuong-Josses France, cont Livrozet Morlat Philibert Pialoux Yazdanpanah Germany Arasteh Bogner Faetkenheuer Hillenbrand Lutz Rockstroh Stellbrink Stephan Stoehr Stoll Italy Castelli Lazzarin D’Arminio Monforte Maggiolo Rizzardini Netherlands Hollander Van Der Ende Russia Belonosova Kulagin Nagimova Pokrovsky Shuldyakov Sultanov Tsybakova Voronin Zakharova Spain Antela Barros Bernal Morell Castro Iglesias Dronda Nuñez Falco Ferrer Fernandez Flores Cid Garcia Deltoro Garcia Gasalla Gomez Sirvent Gonzalez Garcia Gorgolas Hernandez-Mora Hernandez-Quero Knobel Llibre Masia Merino Muñoz Olalla Sierra Spain, cont Palter Peñaranda Vera Pineda Podzamczer Reus Bañuls Rubio Santos Sanz Moreno Telenti Vera Mendez Viciana Taiwan Hung Lin Tsai Wong Yang USA Bares Baxter Bettacchi Bredeek Brennan Brinson Crofoot Dejesus Drelichman Felizarta Goldstein Hagins Katner Kumar Lalezari Mills Osiyemi USA, cont Parks Pierone Ramgopal Richmond Ruane Schneider Shalit Shon Simon Win Wohl United Kingdom Johnson Orkin Ustianowski – The Vii. V Healthcare, GSK, and Janssen study teams This study was funded by Vii. V Healthcare. Llibre et al. CROI 2017; Seattle, WA. Abstract 2421. Conference on Retroviruses and Opportunistic Infections; February 13 -16, 2017; Seattle, WA