Switch to INSTI NNRTI Switch to DTG RPV

- Slides: 11

Switch to INSTI + NNRTI § Switch to DTG + RPV ‒ SWORD Study § Switch to CAB LA + RPV LA IM ‒ LATTE-2 Study

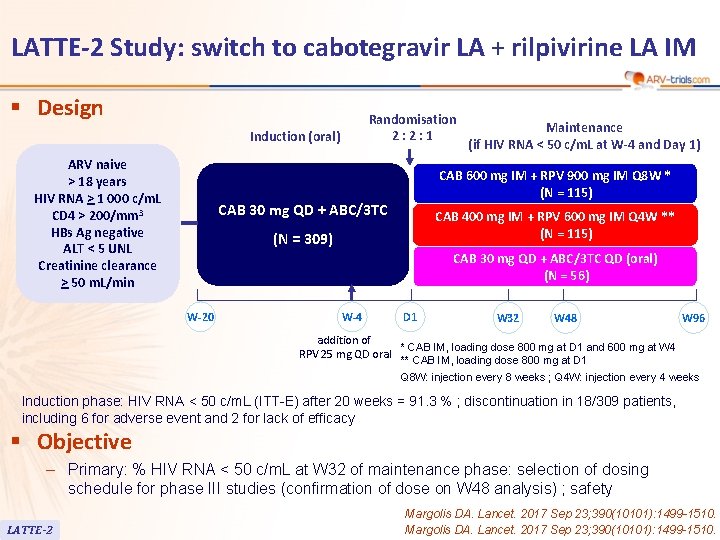
LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM § Design Randomisation 2: 2: 1 Induction (oral) ARV naive > 18 years HIV RNA > 1 000 c/m. L CD 4 > 200/mm 3 HBs Ag negative ALT < 5 UNL Creatinine clearance > 50 m. L/min Maintenance (if HIV RNA < 50 c/m. L at W-4 and Day 1) CAB 600 mg IM + RPV 900 mg IM Q 8 W * (N = 115) CAB 30 mg QD + ABC/3 TC CAB 400 mg IM + RPV 600 mg IM Q 4 W ** (N = 115) (N = 309) CAB 30 mg QD + ABC/3 TC QD (oral) (N = 56) W-20 W-4 D 1 W 32 W 48 W 96 addition of * CAB IM, loading dose 800 mg at D 1 and 600 mg at W 4 RPV 25 mg QD oral ** CAB IM, loading dose 800 mg at D 1 Q 8 W: injection every 8 weeks ; Q 4 W: injection every 4 weeks Induction phase: HIV RNA < 50 c/m. L (ITT-E) after 20 weeks = 91. 3 % ; discontinuation in 18/309 patients, including 6 for adverse event and 2 for lack of efficacy § Objective – Primary: % HIV RNA < 50 c/m. L at W 32 of maintenance phase: selection of dosing schedule for phase III studies (confirmation of dose on W 48 analysis) ; safety LATTE-2 Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.

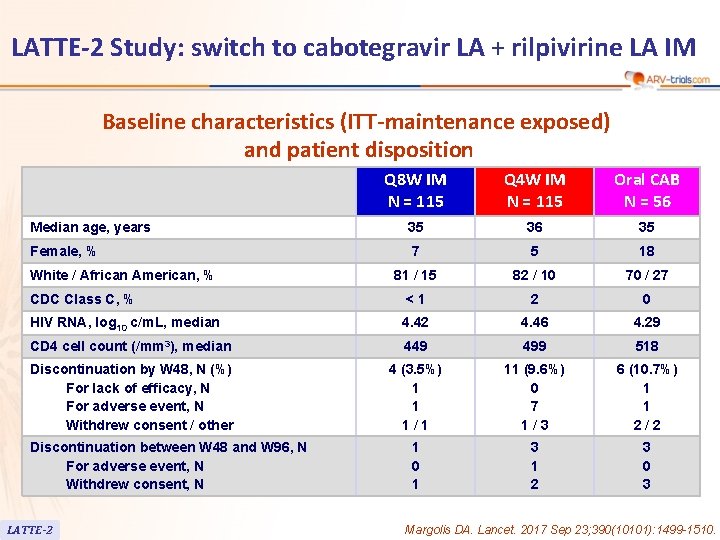
LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM Baseline characteristics (ITT-maintenance exposed) and patient disposition Q 8 W IM N = 115 Q 4 W IM N = 115 Oral CAB N = 56 Median age, years 35 36 35 Female, % 7 5 18 81 / 15 82 / 10 70 / 27 CDC Class C, % <1 2 0 HIV RNA, log 10 c/m. L, median 4. 42 4. 46 4. 29 CD 4 cell count (/mm 3), median 449 499 518 Discontinuation by W 48, N (%) For lack of efficacy, N For adverse event, N Withdrew consent / other 4 (3. 5%) 1 1 1/1 11 (9. 6%) 0 7 1/3 6 (10. 7%) 1 1 2/2 1 0 1 3 1 2 3 0 3 White / African American, % Discontinuation between W 48 and W 96, N For adverse event, N Withdrew consent, N LATTE-2 Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.

LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM Primary endpoint: HIV RNA < 50 c/m. L at W 32 (snapshot analysis, ITT-ME) 100 % 95 94 91 Q 8 W IM (N = 115) Q 4 W IM (N = 115) CAB oral (N = 56) 80 Difference (95% CI) Oral Intramuscular Q 8 W 60 3. 7 - 4. 8 40 12. 2 ‒ 10% 20 4 0 Virologic success <1 4 Virologic Non response <1 5 + 10% Q 4 W 5 2. 8 No virologic data 11. 5 - 5. 8 ‒ 10% 0 + 10% Non inferiority of the 2 IM regimens vs oral CAB LATTE-2 Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.

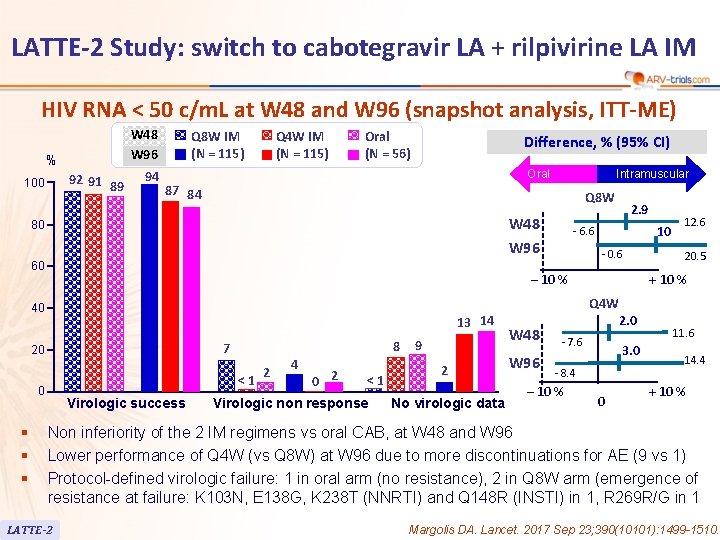
LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM HIV RNA < 50 c/m. L at W 48 and W 96 (snapshot analysis, ITT-ME) W 48 W 96 % 92 91 89 100 94 Q 8 W IM (N = 115) Q 4 W IM (N = 115) Oral (N = 56) Difference, % (95% CI) Oral Intramuscular 87 84 Q 8 W W 48 80 - 0. 6 ‒ 10 % 40 13 14 8 7 20 <1 0 § § § 10 - 6. 6 W 96 60 2. 9 Virologic success 2 4 0 2 <1 Virologic non response 9 2 No virologic data 12. 6 20. 5 + 10 % Q 4 W W 48 W 96 2. 0 - 7. 6 3. 0 - 8. 4 ‒ 10 % 0 11. 6 14. 4 + 10 % Non inferiority of the 2 IM regimens vs oral CAB, at W 48 and W 96 Lower performance of Q 4 W (vs Q 8 W) at W 96 due to more discontinuations for AE (9 vs 1) Protocol-defined virologic failure: 1 in oral arm (no resistance), 2 in Q 8 W arm (emergence of resistance at failure: K 103 N, E 138 G, K 238 T (NNRTI) and Q 148 R (INSTI) in 1, R 269 R/G in 1 LATTE-2 Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.

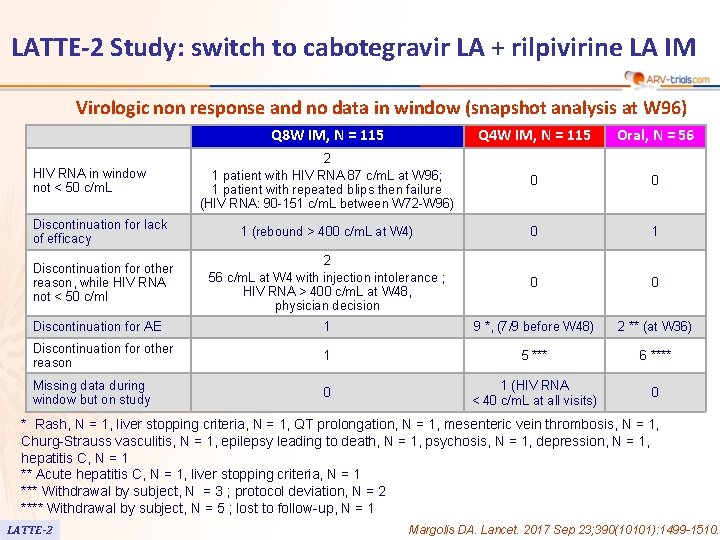
LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM Virologic non response and no data in window (snapshot analysis at W 96) Q 8 W IM, N = 115 Q 4 W IM, N = 115 Oral, N = 56 2 1 patient with HIV RNA 87 c/m. L at W 96; 1 patient with repeated blips then failure (HIV RNA: 90 -151 c/m. L between W 72 -W 96) 0 0 Discontinuation for lack of efficacy 1 (rebound > 400 c/m. L at W 4) 0 1 Discontinuation for other reason, while HIV RNA not < 50 c/ml 2 56 c/m. L at W 4 with injection intolerance ; HIV RNA > 400 c/m. L at W 48, physician decision 0 0 Discontinuation for AE 1 9 *, (7/9 before W 48) 2 ** (at W 36) Discontinuation for other reason 1 5 *** 6 **** Missing data during window but on study 0 1 (HIV RNA < 40 c/m. L at all visits) 0 HIV RNA in window not < 50 c/m. L * Rash, N = 1, liver stopping criteria, N = 1, QT prolongation, N = 1, mesenteric vein thrombosis, N = 1, Churg-Strauss vasculitis, N = 1, epilepsy leading to death, N = 1, psychosis, N = 1, depression, N = 1, hepatitis C, N = 1 ** Acute hepatitis C, N = 1, liver stopping criteria, N = 1 *** Withdrawal by subject, N = 3 ; protocol deviation, N = 2 **** Withdrawal by subject, N = 5 ; lost to follow-up, N = 1 LATTE-2 Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.

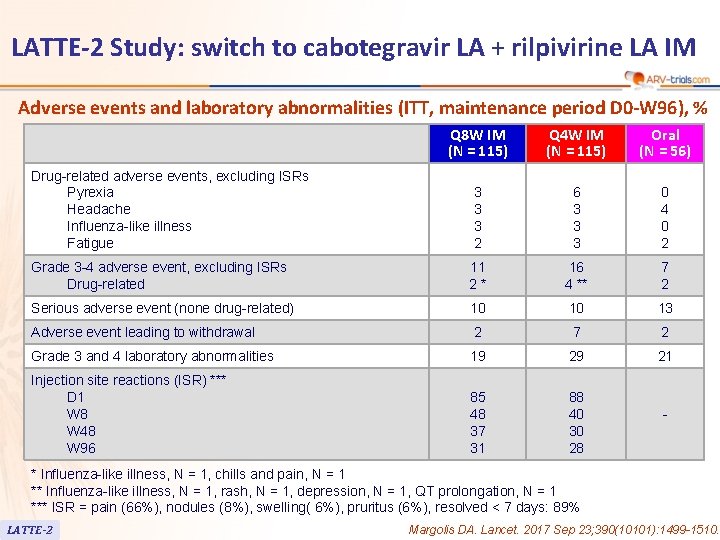
LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM Adverse events and laboratory abnormalities (ITT, maintenance period D 0 -W 96), % Q 8 W IM (N = 115) Q 4 W IM (N = 115) Oral (N = 56) 3 3 3 2 6 3 3 3 0 4 0 2 Grade 3 -4 adverse event, excluding ISRs Drug-related 11 2* 16 4 ** 7 2 Serious adverse event (none drug-related) 10 10 13 Adverse event leading to withdrawal 2 7 2 Grade 3 and 4 laboratory abnormalities 19 29 21 Injection site reactions (ISR) *** D 1 W 8 W 48 W 96 85 48 37 31 88 40 30 28 Drug-related adverse events, excluding ISRs Pyrexia Headache Influenza-like illness Fatigue - * Influenza-like illness, N = 1, chills and pain, N = 1 ** Influenza-like illness, N = 1, rash, N = 1, depression, N = 1, QT prolongation, N = 1 *** ISR = pain (66%), nodules (8%), swelling( 6%), pruritus (6%), resolved < 7 days: 89% LATTE-2 Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.

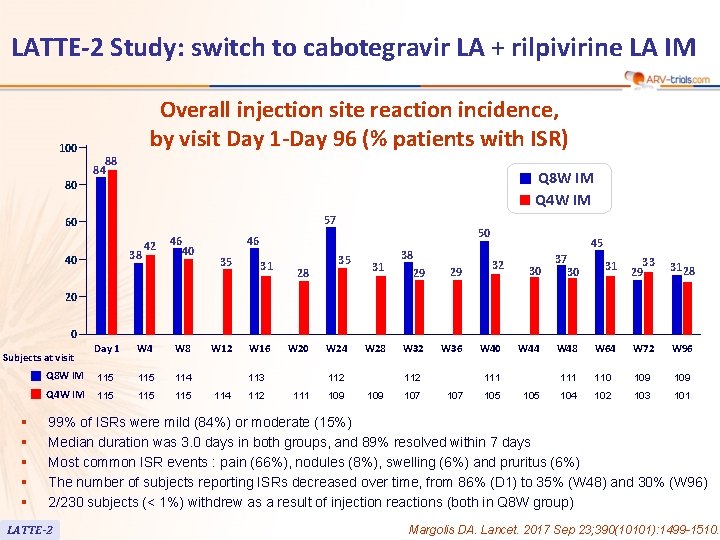
LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM 100 Overall injection site reaction incidence, by visit Day 1 -Day 96 (% patients with ISR) 88 84 Q 8 W IM Q 4 W IM 80 57 60 38 40 42 46 40 50 46 35 31 W 12 W 16 28 35 31 38 29 29 W 28 W 32 W 36 45 32 30 37 31 33 29 31 28 W 48 W 64 W 72 W 96 111 110 109 104 102 103 101 30 20 0 Day 1 W 4 W 8 Q 8 W IM 115 114 Q 4 W IM 115 115 Subjects at visit § § § W 20 113 114 112 W 24 112 111 109 112 109 107 W 40 W 44 111 107 105 99% of ISRs were mild (84%) or moderate (15%) Median duration was 3. 0 days in both groups, and 89% resolved within 7 days Most common ISR events : pain (66%), nodules (8%), swelling (6%) and pruritus (6%) The number of subjects reporting ISRs decreased over time, from 86% (D 1) to 35% (W 48) and 30% (W 96) 2/230 subjects (< 1%) withdrew as a result of injection reactions (both in Q 8 W group) LATTE-2 Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.

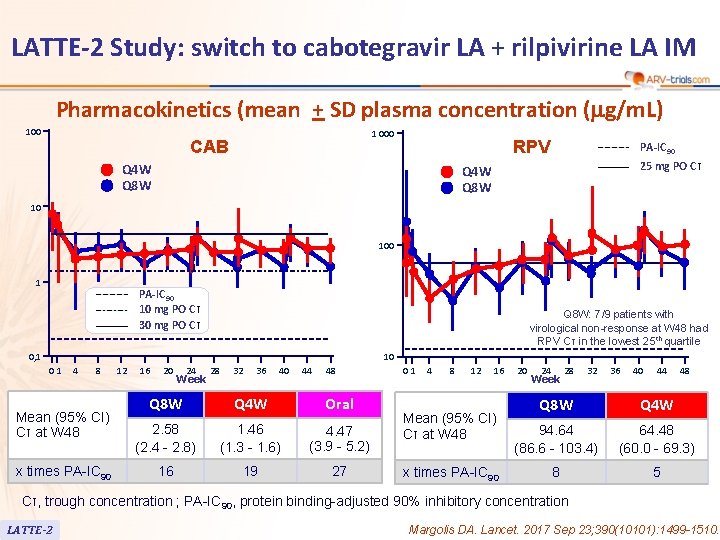
LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM Pharmacokinetics (mean + SD plasma concentration (mg/m. L) 100 1 000 CAB RPV Q 4 W Q 8 W PA-IC 90 25 mg PO Cτ Q 4 W Q 8 W 10 100 1 PA-IC 90 10 mg PO Cτ 30 mg PO Cτ Q 8 W: 7/9 patients with virological non-response at W 48 had RPV Cτ in the lowest 25 th quartile 0, 1 10 01 4 8 Mean (95% CI) Cτ at W 48 x times PA-IC 90 12 16 20 24 Week 28 32 36 40 44 48 Q 8 W Q 4 W Oral 2. 58 (2. 4 - 2. 8) 1. 46 (1. 3 - 1. 6) 4. 47 (3. 9 - 5. 2) 16 19 27 01 4 8 12 16 Mean (95% CI) Cτ at W 48 x times PA-IC 90 20 24 Week 28 32 36 40 44 48 Q 8 W Q 4 W 94. 64 (86. 6 - 103. 4) 64. 48 (60. 0 - 69. 3) 8 5 Cτ, trough concentration ; PA-IC 90, protein binding-adjusted 90% inhibitory concentration LATTE-2 Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.

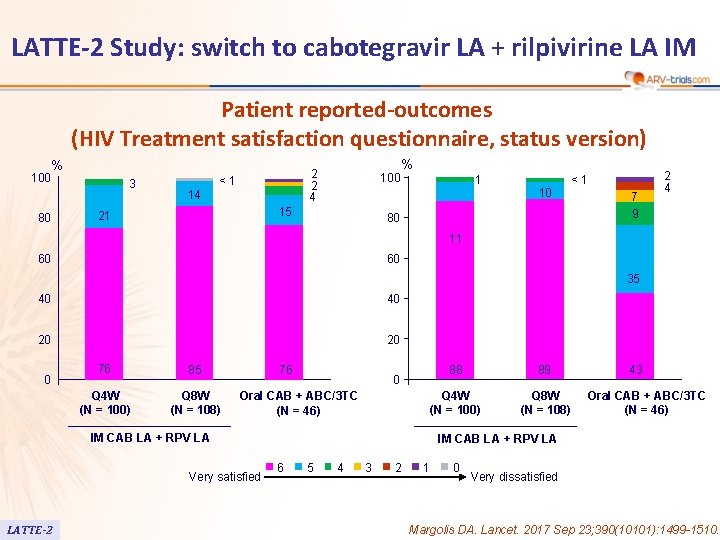
LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM Patient reported-outcomes (HIV Treatment satisfaction questionnaire, status version) % 100 80 3 2 2 4 <1 14 100 15 21 % 1 <1 10 80 7 9 2 4 11 60 60 35 40 40 20 20 0 76 85 Q 4 W (N = 100) Q 8 W (N = 108) 76 0 Oral CAB + ABC/3 TC (N = 46) IM CAB LA + RPV LA Very satisfied LATTE-2 88 89 Q 4 W (N = 100) Q 8 W (N = 108) 43 Oral CAB + ABC/3 TC (N = 46) IM CAB LA + RPV LA 6 5 4 3 2 1 0 Very dissatisfied Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.

LATTE-2 Study: switch to cabotegravir LA + rilpivirine LA IM § Conclusion – LATTE-2 results successfully demonstrate ability to maintain HIV-1 RNA < 50 c/m. L with IM CAB + RPV LA, dosed every 4 or 8 weeks – Three subjects met PDVF criteria during maintenance • Q 8 W (N = 2), oral CAB (N = 1) ; one Q 8 W subject with emergent RPV and CAB resistance, and one Q 8 W subject with minor INSTI mutation emergence – Injection tolerability • Majority of ISRs were grade 1 to 2 pain, with a median duration of 3 days • Few subjects had an ISR that led to discontinuation, with higher rate in Q 4 W group • High overall reported satisfaction – Dose selection • Q 4 W dosing resulted in lower rates of virologic non-response with similar safety to Q 8 W • Q 4 W dosing was selected for pivotal phase III studies LATTE-2 Margolis DA. Lancet. 2017 Sep 23; 390(10101): 1499 -1510.