DTG TAFFTC vs DTG TDFFTC vs EFVTDFFTC ADVANCE

- Slides: 11

DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC § ADVANCE

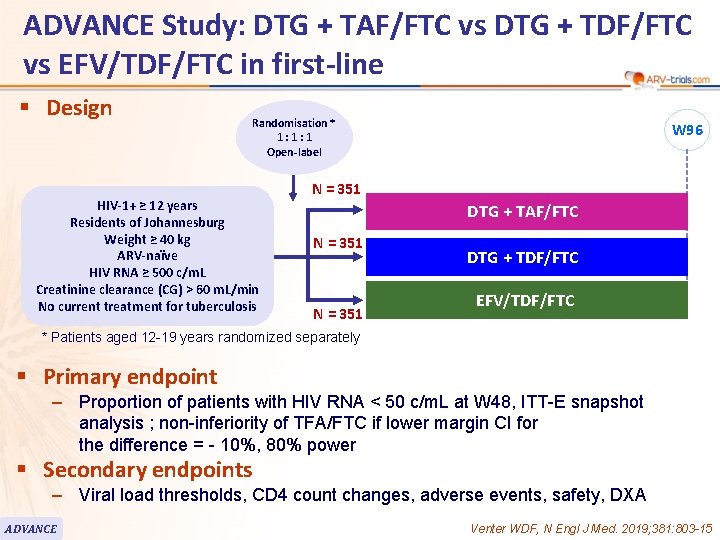
ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line § Design Randomisation * 1: 1: 1 Open-label HIV-1+ ≥ 12 years Residents of Johannesburg Weight ≥ 40 kg ARV-naïve HIV RNA ≥ 500 c/m. L Creatinine clearance (CG) > 60 m. L/min No current treatment for tuberculosis W 96 N = 351 DTG + TAF/FTC N = 351 DTG + TDF/FTC EFV/TDF/FTC * Patients aged 12 -19 years randomized separately § Primary endpoint – Proportion of patients with HIV RNA < 50 c/m. L at W 48, ITT-E snapshot analysis ; non-inferiority of TFA/FTC if lower margin CI for the difference = - 10%, 80% power § Secondary endpoints – Viral load thresholds, CD 4 count changes, adverse events, safety, DXA ADVANCE Venter WDF, N Engl J Med. 2019; 381: 803 -15

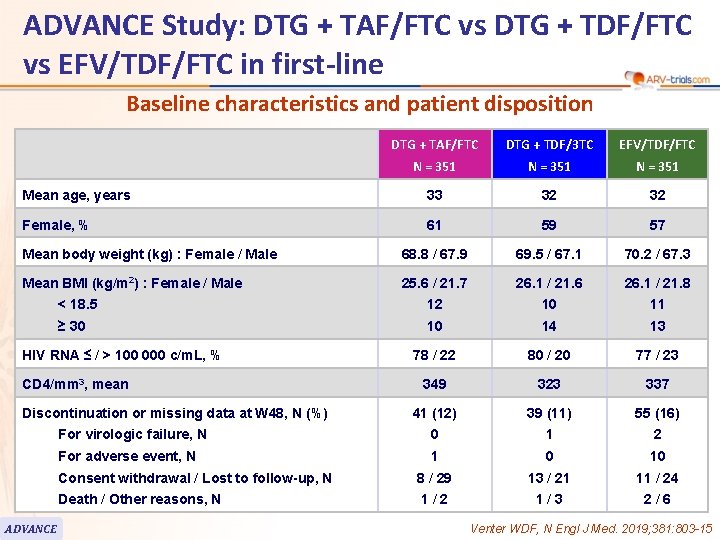
ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line Baseline characteristics and patient disposition DTG + TAF/FTC DTG + TDF/3 TC EFV/TDF/FTC N = 351 Mean age, years 33 32 32 Female, % 61 59 57 Mean body weight (kg) : Female / Male 68. 8 / 67. 9 69. 5 / 67. 1 70. 2 / 67. 3 Mean BMI (kg/m 2) : Female / Male 25. 6 / 21. 7 26. 1 / 21. 6 26. 1 / 21. 8 < 18. 5 12 10 11 ≥ 30 10 14 13 78 / 22 80 / 20 77 / 23 349 323 337 41 (12) 39 (11) 55 (16) For virologic failure, N 0 1 2 For adverse event, N 1 0 10 Consent withdrawal / Lost to follow-up, N 8 / 29 13 / 21 11 / 24 Death / Other reasons, N 1/2 1/3 2/6 HIV RNA ≤ / > 100 000 c/m. L, % CD 4/mm 3, mean Discontinuation or missing data at W 48, N (%) ADVANCE Venter WDF, N Engl J Med. 2019; 381: 803 -15

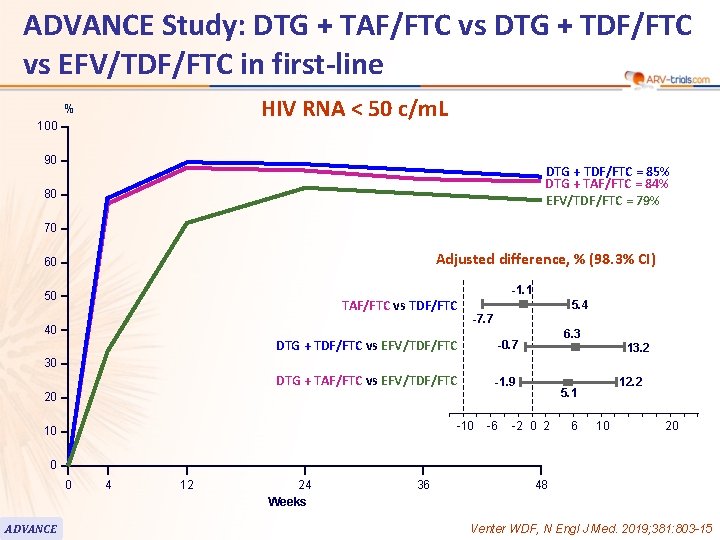
ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line HIV RNA < 50 c/m. L % 100 90 DTG + TDF/FTC = 85% DTG + TAF/FTC = 84% EFV/TDF/FTC = 79% 80 70 Adjusted difference, % (98. 3% CI) 60 -1. 1 50 TAF/FTC vs TDF/FTC 40 5. 4 -7. 7 DTG + TDF/FTC vs EFV/TDF/FTC -0. 7 DTG + TAF/FTC vs EFV/TDF/FTC -1. 9 6. 3 13. 2 30 20 -10 10 -6 12. 2 5. 1 -2 0 2 6 10 20 0 0 ADVANCE 4 12 24 Weeks 36 48 Venter WDF, N Engl J Med. 2019; 381: 803 -15

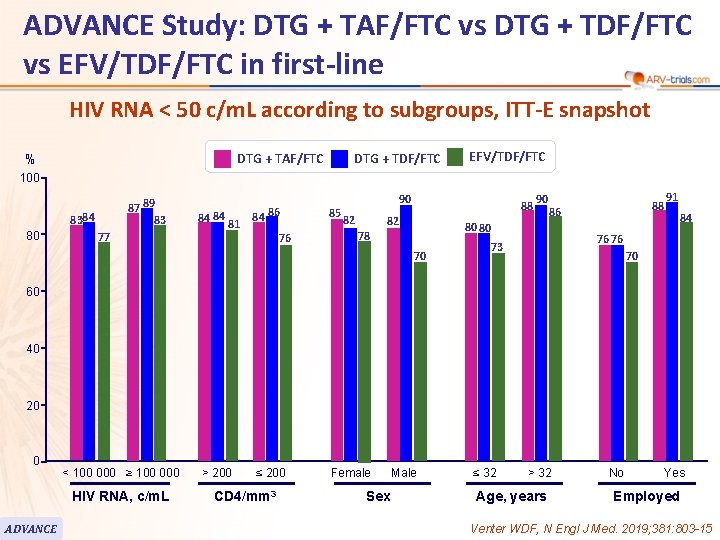
ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line 76 HIV RNA < 50 c/m. L according to subgroups, ITT-E snapshot DTG + TDF/FTC DTG + TAF/FTC % EFV/TDF/FTC 100 87 89 83 8384 80 77 84 84 81 84 86 76 85 90 82 88 82 90 80 80 78 70 88 86 91 84 76 76 73 70 60 40 20 0 < 100 000 ≥ 100 000 HIV RNA, c/m. L ADVANCE > 200 ≤ 200 CD 4/mm 3 Female Sex Male ≤ 32 > 32 Age, years No Yes Employed Venter WDF, N Engl J Med. 2019; 381: 803 -15

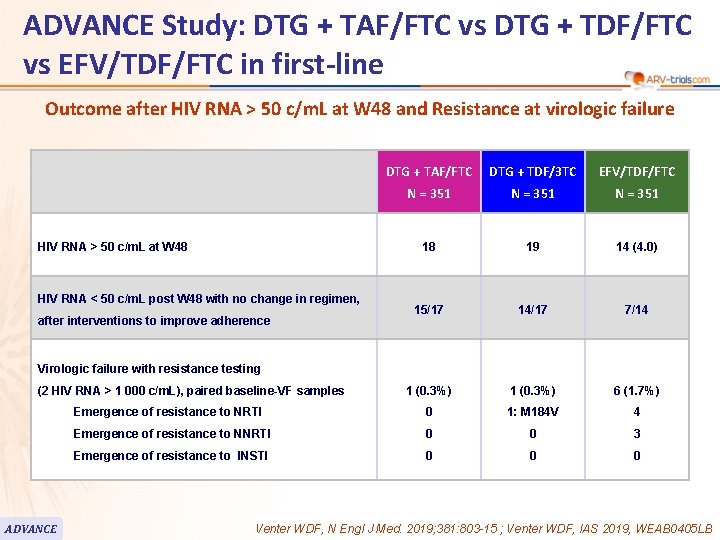
ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line Outcome after HIV RNA > 50 c/m. L at W 48 and Resistance at virologic failure DTG + TAF/FTC DTG + TDF/3 TC EFV/TDF/FTC N = 351 18 19 14 (4. 0) 15/17 14/17 7/14 1 (0. 3%) 6 (1. 7%) Emergence of resistance to NRTI 0 1: M 184 V 4 Emergence of resistance to NNRTI 0 0 3 Emergence of resistance to INSTI 0 0 0 HIV RNA > 50 c/m. L at W 48 HIV RNA < 50 c/m. L post W 48 with no change in regimen, after interventions to improve adherence Virologic failure with resistance testing (2 HIV RNA > 1 000 c/m. L), paired baseline-VF samples ADVANCE Venter WDF, N Engl J Med. 2019; 381: 803 -15 ; Venter WDF, IAS 2019, WEAB 0405 LB

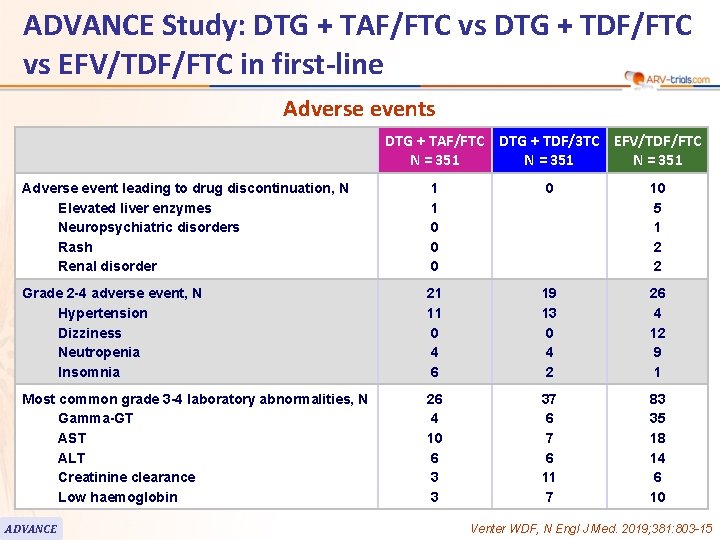
ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line Adverse events DTG + TAF/FTC DTG + TDF/3 TC EFV/TDF/FTC N = 351 Adverse event leading to drug discontinuation, N Elevated liver enzymes Neuropsychiatric disorders Rash Renal disorder 1 1 0 0 10 5 1 2 2 Grade 2 -4 adverse event, N Hypertension Dizziness Neutropenia Insomnia 21 11 0 4 6 19 13 0 4 2 26 4 12 9 1 Most common grade 3 -4 laboratory abnormalities, N Gamma-GT AST ALT Creatinine clearance Low haemoglobin 26 4 10 6 3 3 37 6 11 7 83 35 18 14 6 10 ADVANCE Venter WDF, N Engl J Med. 2019; 381: 803 -15

ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line DXA results at W 48 DTG + TAF/FTC N = 351 DTG + TDF/3 TC N = 351 EFV/TDF/FTC N = 351 Whole body 1. 4 2. 0 0. 8 Spine 18. 2 22. 7 22. 3 Hip 6. 6 16. 2 17. 8 Spine 4. 5 7. 0 7. 7 Hip 1. 3 0. 9 4. 6 Female 19. 5 10. 6 8. 7 Male 7. 1 3. 4 New osteopenia, % New osteoporosis, % New obesity (BMI ≥ 30 kg/m 2), % ADVANCE Venter WDF, N Engl J Med. 2019; 381: 803 -15

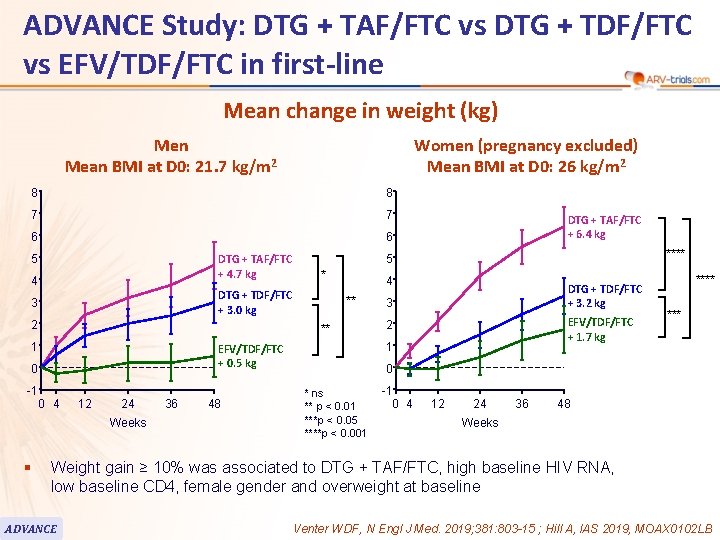
ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line 99 Mean change in weight (kg) Men Mean BMI at D 0: 21. 7 kg/m 2 8 8 7 7 6 6 DTG + TAF/FTC + 4. 7 kg 5 4 2 0 4 12 24 Weeks 36 48 **** 4 ** DTG + TDF/FTC + 3. 2 kg 3 EFV/TDF/FTC + 1. 7 kg 2 1 EFV/TDF/FTC + 0. 5 kg 0 -1 * ** 1 DTG + TAF/FTC + 6. 4 kg 5 DTG + TDF/FTC + 3. 0 kg 3 § Women (pregnancy excluded) Mean BMI at D 0: 26 kg/m 2 **** 0 * ns ** p < 0. 01 ***p < 0. 05 ****p < 0. 001 -1 0 4 12 24 36 48 Weeks Weight gain ≥ 10% was associated to DTG + TAF/FTC, high baseline HIV RNA, low baseline CD 4, female gender and overweight at baseline ADVANCE Venter WDF, N Engl J Med. 2019; 381: 803 -15 ; Hill A, IAS 2019, MOAX 0102 LB

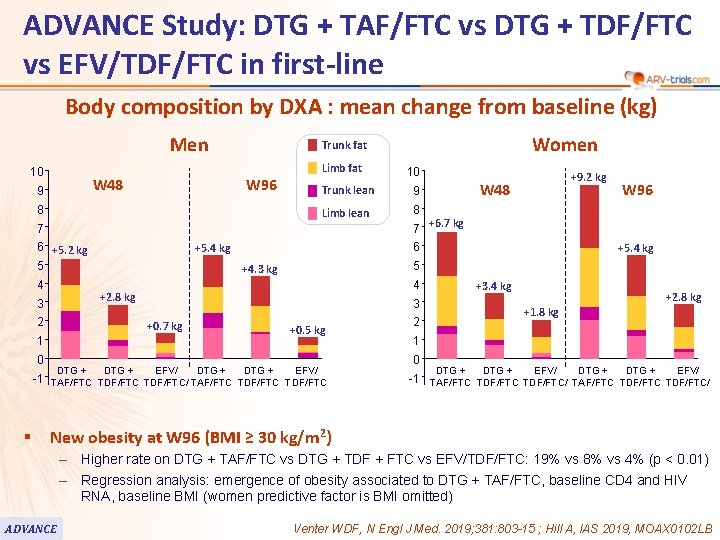
ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line 100 Body composition by DXA : mean change from baseline (kg) Men 10 W 48 9 W 96 8 Limb fat 4 9 Limb lean 8 +5. 4 kg 2 1 0 -1 § W 96 7 +6. 7 kg +5. 4 kg 5 +4. 3 kg 4 +3. 4 kg 3 +0. 7 kg +9. 2 kg W 48 6 +2. 8 kg 3 10 Trunk lean 7 6 +5. 2 kg 5 Women Trunk fat +0. 5 kg DTG + EFV/ TAF/FTC TDF/FTC/ TAF/FTC TDF/FTC +2. 8 kg +1. 8 kg 2 1 0 DTG + EFV/ -1 TAF/FTC TDF/FTC/ New obesity at W 96 (BMI ≥ 30 kg/m 2) – Higher rate on DTG + TAF/FTC vs DTG + TDF + FTC vs EFV/TDF/FTC: 19% vs 8% vs 4% (p < 0. 01) – Regression analysis: emergence of obesity associated to DTG + TAF/FTC, baseline CD 4 and HIV RNA, baseline BMI (women predictive factor is BMI omitted) ADVANCE Venter WDF, N Engl J Med. 2019; 381: 803 -15 ; Hill A, IAS 2019, MOAX 0102 LB

ADVANCE Study: DTG + TAF/FTC vs DTG + TDF/FTC vs EFV/TDF/FTC in first-line § Conclusion – Treatment with DTG combined with FTC and either of two tenofovir prodrugs (TAF and TDF) showed noninferior efficacy to treatment with the standard-care regimen of EFV/TDF/FTC – There was significantly more weight gain with the DTG-containing regimens, especially in combination with TAF, than with the standard-care regimen – The concern around the potential teratogenicity of DTG and a dearth of pregnancy safety data with TAF pose complex challenges for practitioners in low- and middle-income countries that rely on health systems with limited options, especially for women – The increased risk of weight gain with both DTG-containing regimens and the limited knowledge base regarding TAF in pregnancy need to be evaluated against improvements in side effect profile and adherence, slight reductions in time to virologic control, and effect on bone mineral density and renal function ADVANCE Venter WDF, N Engl J Med. 2019; 381: 803 -15