Switch to DTG RPV Switch to DTG RPV

- Slides: 11

Switch to DTG + RPV § Switch to DTG + RPV ‒ SWORD Study § Switch to CAB LA + RPV LA IM ‒ LATTE-2 Study

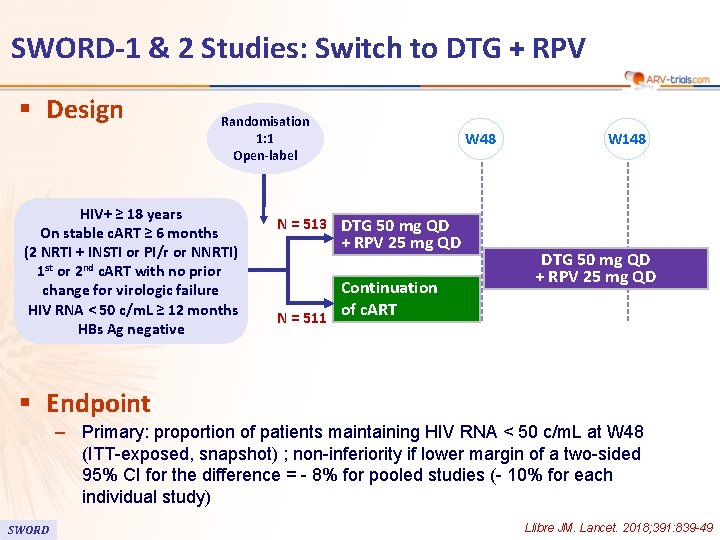
SWORD-1 & 2 Studies: Switch to DTG + RPV § Design Randomisation 1: 1 Open-label HIV+ ≥ 18 years On stable c. ART ≥ 6 months (2 NRTI + INSTI or PI/r or NNRTI) 1 st or 2 nd c. ART with no prior change for virologic failure HIV RNA < 50 c/m. L ≥ 12 months HBs Ag negative W 48 W 148 N = 513 DTG 50 mg QD + RPV 25 mg QD Continuation of c. ART N = 511 DTG 50 mg QD + RPV 25 mg QD § Endpoint – Primary: proportion of patients maintaining HIV RNA < 50 c/m. L at W 48 (ITT-exposed, snapshot) ; non-inferiority if lower margin of a two-sided 95% CI for the difference = - 8% for pooled studies (- 10% for each individual study) SWORD Llibre JM. Lancet. 2018; 391: 839 -49

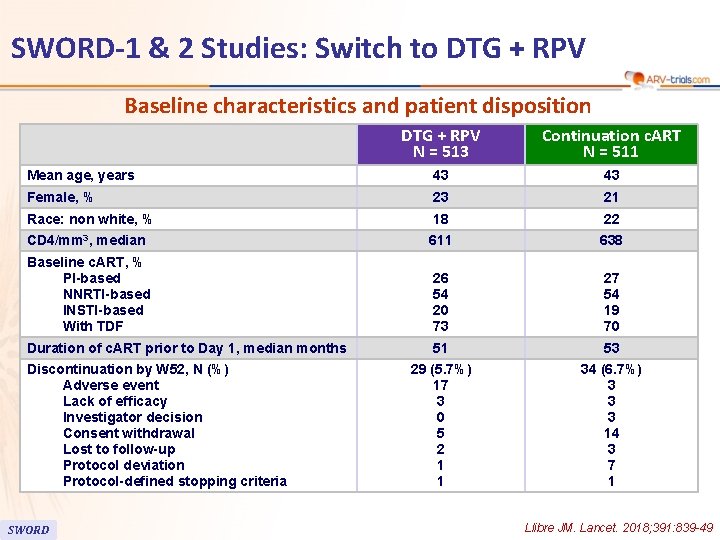
SWORD-1 & 2 Studies: Switch to DTG + RPV Baseline characteristics and patient disposition DTG + RPV N = 513 Continuation c. ART N = 511 Mean age, years 43 43 Female, % 23 21 Race: non white, % 18 22 CD 4/mm 3, median 611 638 Baseline c. ART, % PI-based NNRTI-based INSTI-based With TDF 26 54 20 73 27 54 19 70 Duration of c. ART prior to Day 1, median months 51 53 29 (5. 7%) 17 3 0 5 2 1 1 34 (6. 7%) 3 3 3 14 3 7 1 Discontinuation by W 52, N (%) Adverse event Lack of efficacy Investigator decision Consent withdrawal Lost to follow-up Protocol deviation Protocol-defined stopping criteria SWORD Llibre JM. Lancet. 2018; 391: 839 -49

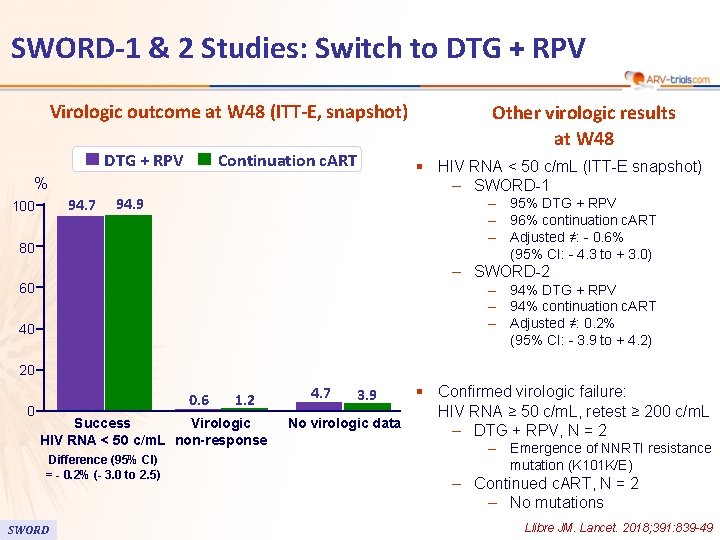
SWORD-1 & 2 Studies: Switch to DTG + RPV Virologic outcome at W 48 (ITT-E, snapshot) DTG + RPV Continuation c. ART § HIV RNA < 50 c/m. L (ITT-E snapshot) ‒ SWORD-1 % 94. 7 100 Other virologic results at W 48 94. 9 ‒ 95% DTG + RPV ‒ 96% continuation c. ART ‒ Adjusted ≠: - 0. 6% (95% CI: - 4. 3 to + 3. 0) 80 ‒ SWORD-2 60 ‒ 94% DTG + RPV ‒ 94% continuation c. ART ‒ Adjusted ≠: 0. 2% (95% CI: - 3. 9 to + 4. 2) 40 20 0 0. 6 1. 2 Success Virologic HIV RNA < 50 c/m. L non-response Difference (95% CI) = - 0. 2% (- 3. 0 to 2. 5) SWORD § Confirmed virologic failure: HIV RNA ≥ 50 c/m. L, retest ≥ 200 c/m. L No virologic data ‒ DTG + RPV, N = 2 4. 7 3. 9 ‒ Emergence of NNRTI resistance mutation (K 101 K/E) ‒ Continued c. ART, N = 2 ‒ No mutations Llibre JM. Lancet. 2018; 391: 839 -49

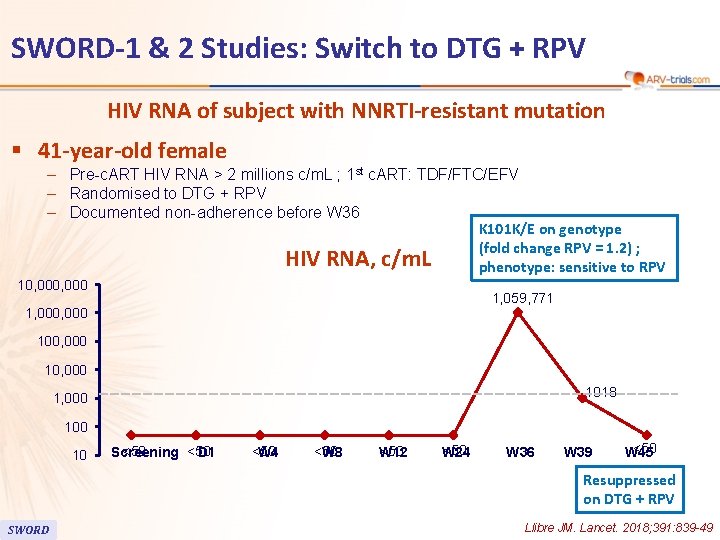
80 SWORD-1 & 2 Studies: Switch to DTG + RPV HIV RNA of subject with NNRTI-resistant mutation § 41 -year-old female – Pre-c. ART HIV RNA > 2 millions c/m. L ; 1 st c. ART: TDF/FTC/EFV – Randomised to DTG + RPV – Documented non-adherence before W 36 K 101 K/E on genotype (fold change RPV = 1. 2) ; HIV RNA, c/m. L phenotype: sensitive to RPV 10, 000 1, 059, 771 1, 000 100, 000 1018 1, 000 10 <50 Screening D 1 <50 W 4 <50 W 8 <50 W 12 <50 W 24 W 36 W 39 <50 W 45 Resuppressed on DTG + RPV SWORD Llibre JM. Lancet. 2018; 391: 839 -49

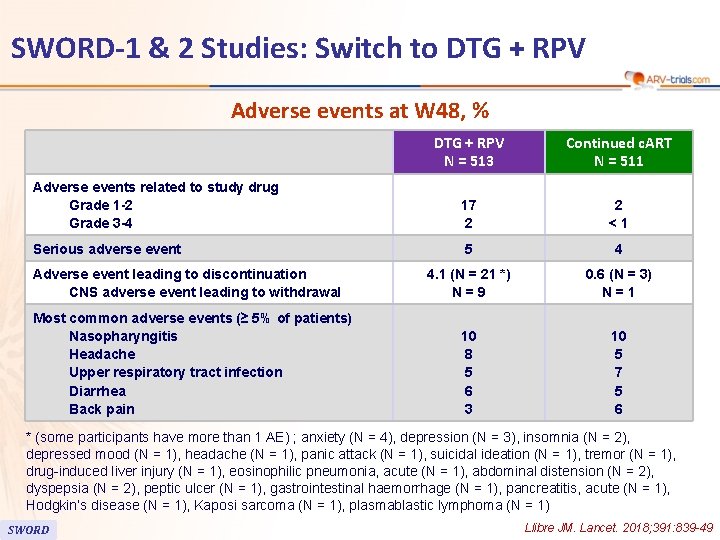
SWORD-1 & 2 Studies: Switch to DTG + RPV Adverse events at W 48, % DTG + RPV N = 513 Continued c. ART N = 511 Adverse events related to study drug Grade 1 -2 Grade 3 -4 17 2 2 <1 Serious adverse event 5 4 4. 1 (N = 21 *) N=9 0. 6 (N = 3) N=1 10 8 5 6 3 10 5 7 5 6 Adverse event leading to discontinuation CNS adverse event leading to withdrawal Most common adverse events (≥ 5% of patients) Nasopharyngitis Headache Upper respiratory tract infection Diarrhea Back pain * (some participants have more than 1 AE) ; anxiety (N = 4), depression (N = 3), insomnia (N = 2), depressed mood (N = 1), headache (N = 1), panic attack (N = 1), suicidal ideation (N = 1), tremor (N = 1), drug-induced liver injury (N = 1), eosinophilic pneumonia, acute (N = 1), abdominal distension (N = 2), dyspepsia (N = 2), peptic ulcer (N = 1), gastrointestinal haemorrhage (N = 1), pancreatitis, acute (N = 1), Hodgkin’s disease (N = 1), Kaposi sarcoma (N = 1), plasmablastic lymphoma (N = 1) SWORD Llibre JM. Lancet. 2018; 391: 839 -49

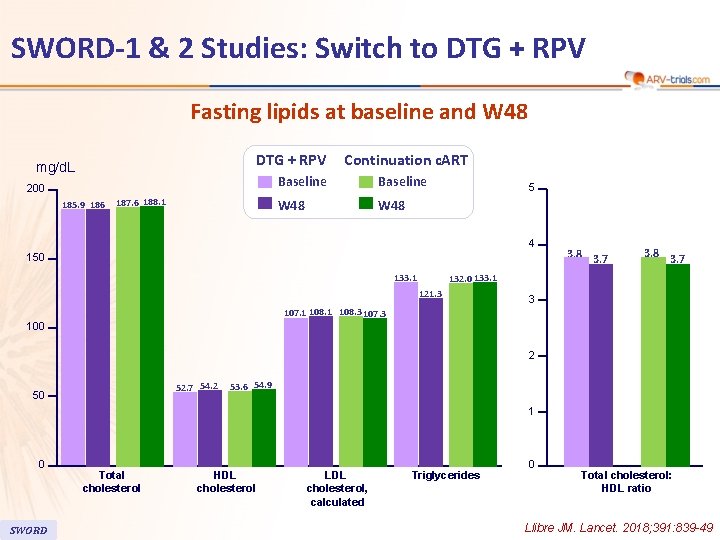
SWORD-1 & 2 Studies: Switch to DTG + RPV Fasting lipids at baseline and W 48 DTG + RPV mg/d. L 200 185. 9 186 187. 6 188. 1 Continuation c. ART Baseline W 48 5 4 150 133. 1 3. 8 3. 7 132. 0 133. 1 121. 3 3 107. 1 108. 3 107. 3 100 2 52. 7 54. 2 50 53. 6 54. 9 1 0 SWORD Total cholesterol HDL cholesterol LDL cholesterol, calculated Triglycerides 0 Total cholesterol: HDL ratio Llibre JM. Lancet. 2018; 391: 839 -49

SWORD-1 & 2 Studies: Switch to DTG + RPV § Conclusion at W 48 – A switch to a novel, once-daily 2 drug-regimen of DTG + RPV demonstrated high efficacy and was non-inferior to the continuation of a combined antiretroviral therapy in virologically suppressed HIV-1–infected adults – The safety profiles of both DTG and RPV were consistent with their respective labels – Switching to DTG + RPV had a neutral effect on lipids, while significantly improving bone turnover biomarkers SWORD Llibre JM. Lancet. 2018; 391: 839 -49

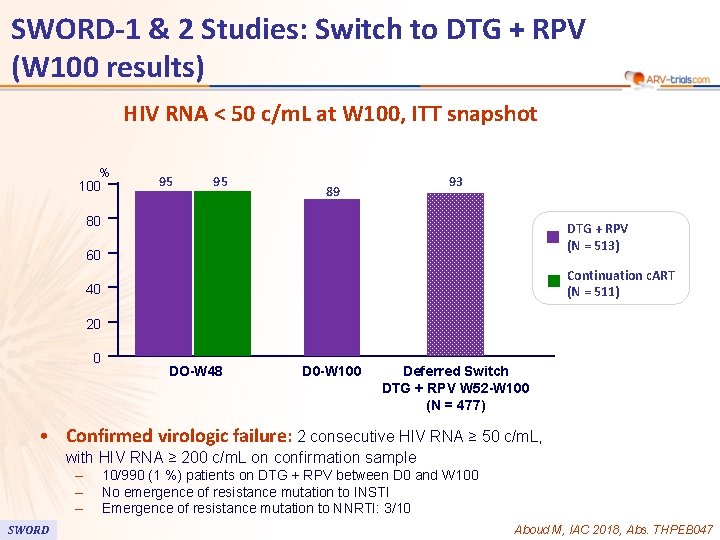
SWORD-1 & 2 Studies: Switch to DTG + RPV (W 100 results) 90 HIV RNA < 50 c/m. L at W 100, ITT snapshot % 100 95 95 93 89 80 DTG + RPV (N = 513) 60 Continuation c. ART (N = 511) 40 20 0 DO-W 48 D 0 -W 100 Deferred Switch DTG + RPV W 52 -W 100 (N = 477) • Confirmed virologic failure: 2 consecutive HIV RNA ≥ 50 c/m. L, with HIV RNA ≥ 200 c/m. L on confirmation sample – – – SWORD 10/990 (1 %) patients on DTG + RPV between D 0 and W 100 No emergence of resistance mutation to INSTI Emergence of resistance mutation to NNRTI: 3/10 Aboud M, IAC 2018, Abs. THPEB 047

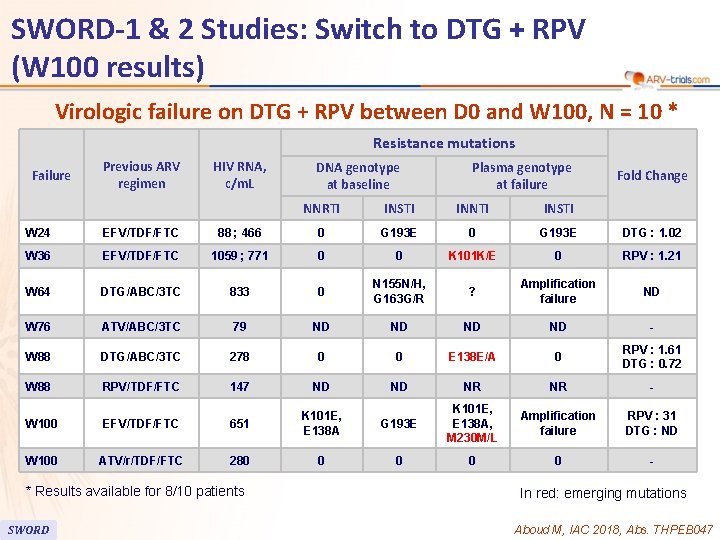
91 SWORD-1 & 2 Studies: Switch to DTG + RPV (W 100 results) Virologic failure on DTG + RPV between D 0 and W 100, N = 10 * Resistance mutations Failure Previous ARV regimen HIV RNA, c/m. L DNA genotype at baseline Plasma genotype at failure NNRTI INSTI INNTI INSTI Fold Change W 24 EFV/TDF/FTC 88 ; 466 0 G 193 E DTG : 1. 02 W 36 EFV/TDF/FTC 1059 ; 771 0 0 K 101 K/E 0 RPV : 1. 21 W 64 DTG/ABC/3 TC 833 0 N 155 N/H, G 163 G/R ? Amplification failure ND W 76 ATV/ABC/3 TC 79 ND ND - W 88 DTG/ABC/3 TC 278 0 0 E 138 E/A 0 RPV : 1. 61 DTG : 0. 72 W 88 RPV/TDF/FTC 147 ND ND NR NR - W 100 EFV/TDF/FTC 651 K 101 E, E 138 A G 193 E K 101 E, E 138 A, M 230 M/L Amplification failure RPV : 31 DTG : ND W 100 ATV/r/TDF/FTC 280 0 0 - * Results available for 8/10 patients SWORD In red: emerging mutations Aboud M, IAC 2018, Abs. THPEB 047

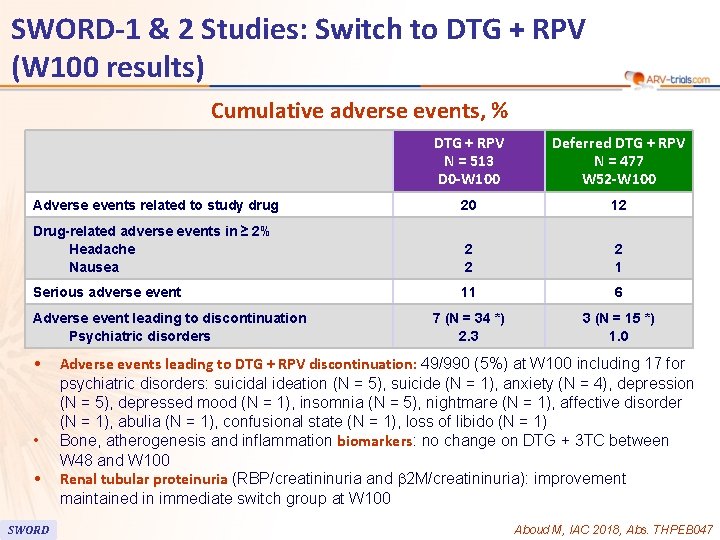
90 SWORD-1 & 2 Studies: Switch to DTG + RPV (W 100 results) Cumulative adverse events, % DTG + RPV N = 513 D 0 -W 100 Deferred DTG + RPV N = 477 W 52 -W 100 Adverse events related to study drug 20 12 Drug-related adverse events in ≥ 2% Headache Nausea 2 2 2 1 Serious adverse event 11 6 7 (N = 34 *) 2. 3 3 (N = 15 *) 1. 0 Adverse event leading to discontinuation Psychiatric disorders • • • SWORD Adverse events leading to DTG + RPV discontinuation: 49/990 (5%) at W 100 including 17 for psychiatric disorders: suicidal ideation (N = 5), suicide (N = 1), anxiety (N = 4), depression (N = 5), depressed mood (N = 1), insomnia (N = 5), nightmare (N = 1), affective disorder (N = 1), abulia (N = 1), confusional state (N = 1), loss of libido (N = 1) Bone, atherogenesis and inflammation biomarkers: no change on DTG + 3 TC between W 48 and W 100 Renal tubular proteinuria (RBP/creatininuria and b 2 M/creatininuria): improvement maintained in immediate switch group at W 100 Aboud M, IAC 2018, Abs. THPEB 047