Stoichiometry overview Recall that in stoichiometry the mole



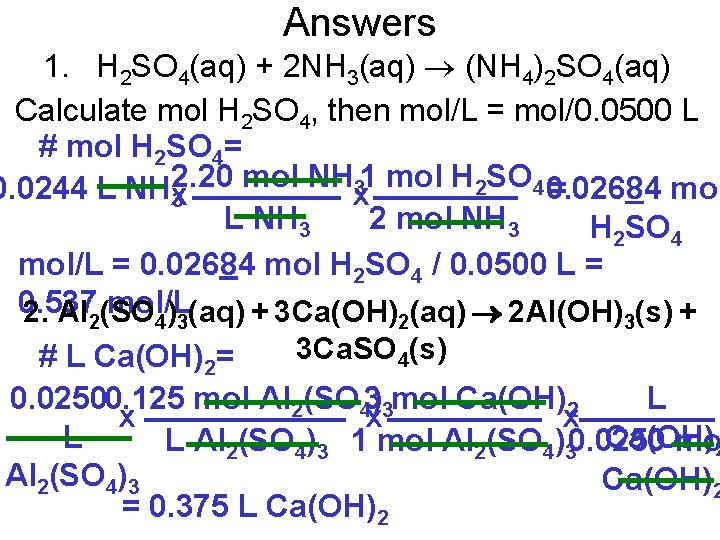


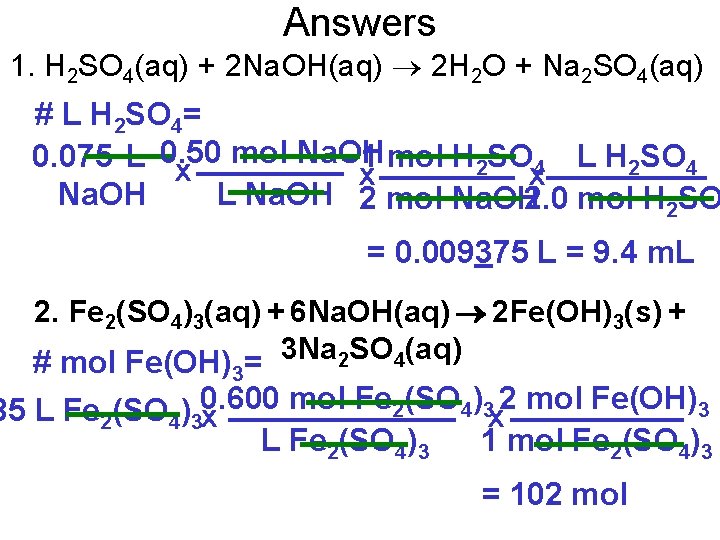
- Slides: 12


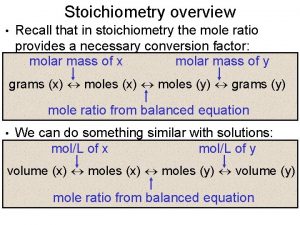
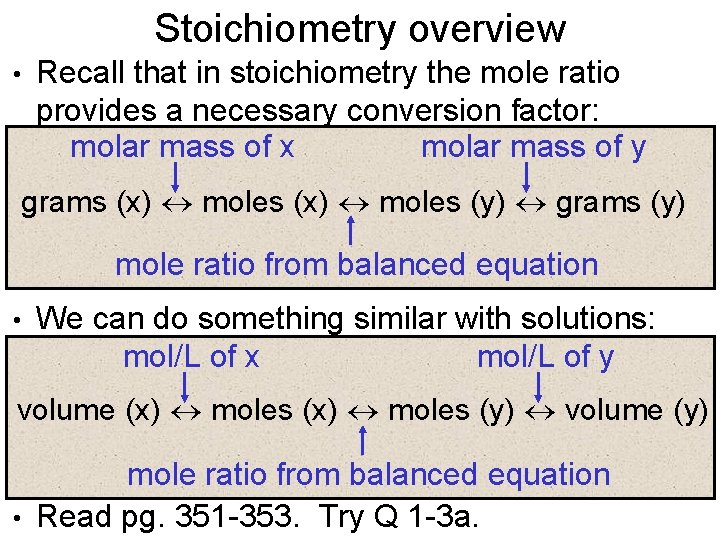
Stoichiometry overview • Recall that in stoichiometry the mole ratio provides a necessary conversion factor: molar mass of x molar mass of y grams (x) moles (y) grams (y) mole ratio from balanced equation • We can do something similar with solutions: mol/L of x mol/L of y volume (x) moles (y) volume (y) mole ratio from balanced equation • Read pg. 351 -353. Try Q 1 -3 a.

Pg. 353, Question 1 Ammonium sulfate is manufactured by reacting sulfuric acid with ammonia. What concentration of sulfuric acid is needed to react with 24. 4 m. L of a 2. 20 mol/L ammonia solution if 50. 0 m. L of sulfuric acid is used? H 2 SO 4(aq) + 2 NH 3(aq) (NH 4)2 SO 4(aq) Calculate mol H 2 SO 4, then mol/L = mol/0. 0500 L # mol H 2 SO 4= 2. 20 mol NH 31 mol H 2 SO 4 0. 02684 0. 0244 L NHx = mo x 3 L NH 3 2 mol NH 3 H 2 SO 4 mol/L = 0. 02684 mol H 2 SO 4 / 0. 0500 L =

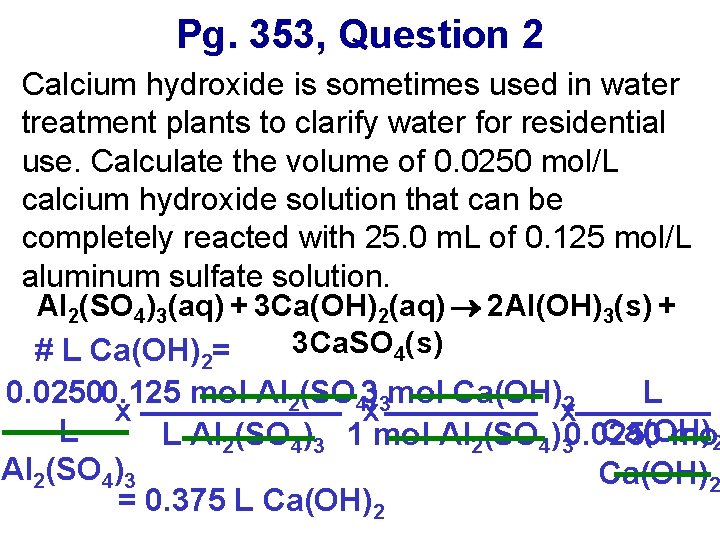
Pg. 353, Question 2 Calcium hydroxide is sometimes used in water treatment plants to clarify water for residential use. Calculate the volume of 0. 0250 mol/L calcium hydroxide solution that can be completely reacted with 25. 0 m. L of 0. 125 mol/L aluminum sulfate solution. Al 2(SO 4)3(aq) + 3 Ca(OH)2(aq) 2 Al(OH)3(s) + 3 Ca. SO 4(s) # L Ca(OH)2= 0. 02500. 125 mol Al 2(SO 43)3 mol Ca(OH)2 L x x x L Ca(OH) L Al 2(SO 4)3 1 mol Al 2(SO 4)30. 0250 mol 2 Al 2(SO 4)3 Ca(OH)2 = 0. 375 L Ca(OH)2

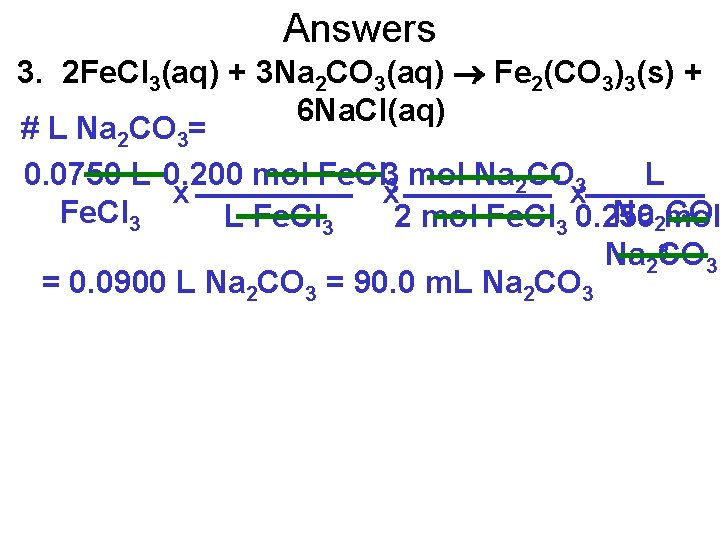
Pg. 353, Question 3 A chemistry teacher wants 75. 0 m. L of 0. 200 mol/L iron(Ill) chloride solution to react completely with an excess of 0. 250 mol/L sodium carbonate solution. What volume of sodium carbonate solution is needed? 2 Fe. Cl 3(aq) + 3 Na 2 CO 3(aq) Fe 2(CO 3)3(s) + 6 Na. Cl(aq) # L Na 2 CO 3= 0. 0750 L 0. 200 mol Fe. Cl 33 mol Na 2 CO 3 L x x x Fe. Cl 3 Na 2 mol CO L Fe. Cl 3 2 mol Fe. Cl 3 0. 250 Na 23 CO 3 = 0. 0900 L Na 2 CO 3 = 90. 0 m. L Na 2 CO 3
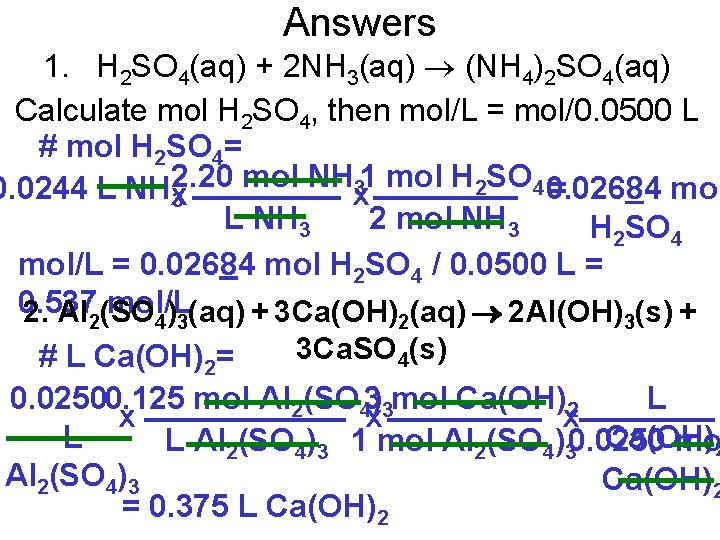
Answers 1. H 2 SO 4(aq) + 2 NH 3(aq) (NH 4)2 SO 4(aq) Calculate mol H 2 SO 4, then mol/L = mol/0. 0500 L # mol H 2 SO 4= 2. 20 mol NH 31 mol H 2 SO 4 0. 02684 0. 0244 L NHx = mol x 3 L NH 3 2 mol NH 3 H 2 SO 4 mol/L = 0. 02684 mol H 2 SO 4 / 0. 0500 L = 0. 537 mol/L 2. Al 2(SO 4)3(aq) + 3 Ca(OH)2(aq) 2 Al(OH)3(s) + 3 Ca. SO 4(s) # L Ca(OH)2= 0. 02500. 125 mol Al 2(SO 43)3 mol Ca(OH)2 L x x x L Ca(OH) L Al 2(SO 4)3 1 mol Al 2(SO 4)30. 0250 mo 2 Al 2(SO 4)3 Ca(OH)2 = 0. 375 L Ca(OH)2

Answers 3. 2 Fe. Cl 3(aq) + 3 Na 2 CO 3(aq) Fe 2(CO 3)3(s) + 6 Na. Cl(aq) # L Na 2 CO 3= 0. 0750 L 0. 200 mol Fe. Cl 33 mol Na 2 CO 3 L x x x Fe. Cl 3 Na 2 mol CO L Fe. Cl 3 2 mol Fe. Cl 3 0. 250 Na 23 CO 3 = 0. 0900 L Na 2 CO 3 = 90. 0 m. L Na 2 CO 3

Assignment 1. H 2 SO 4 reacts with Na. OH, producing water and sodium sulfate. What volume of 2. 0 M H 2 SO 4 will be required to react completely with 75 m. L of 0. 50 mol/L Na. OH? 2. How many moles of Fe(OH)3 are produced when 85. 0 L of iron(III) sulfate at a concentration of 0. 600 mol/L reacts with excess Na. OH? 3. What mass of precipitate will be produced from the reaction of 50. 0 m. L of 2. 50 mol/L sodium hydroxide with an excess of zinc chloride solution.

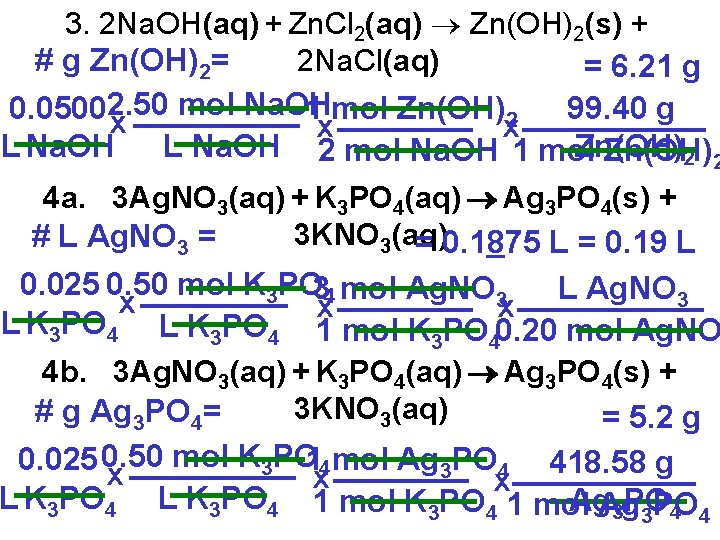
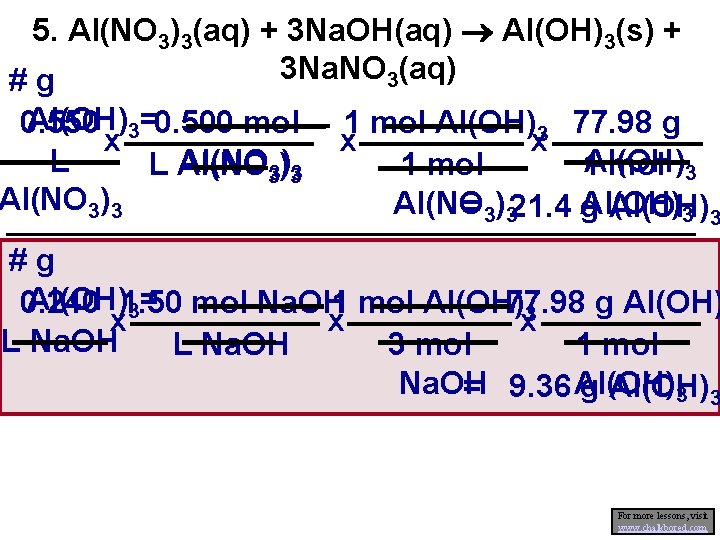
Assignment 4. a) What volume of 0. 20 mol/L Ag. NO 3 will be needed to react completely with 25. 0 m. L of 0. 50 mol/L potassium phosphate? b) What mass of precipitate is produced from the above reaction? 5. What mass of precipitate should result when 0. 550 L of 0. 500 mol/L aluminum nitrate solution is mixed with 0. 240 L of 1. 50 mol/L sodium hydroxide solution?
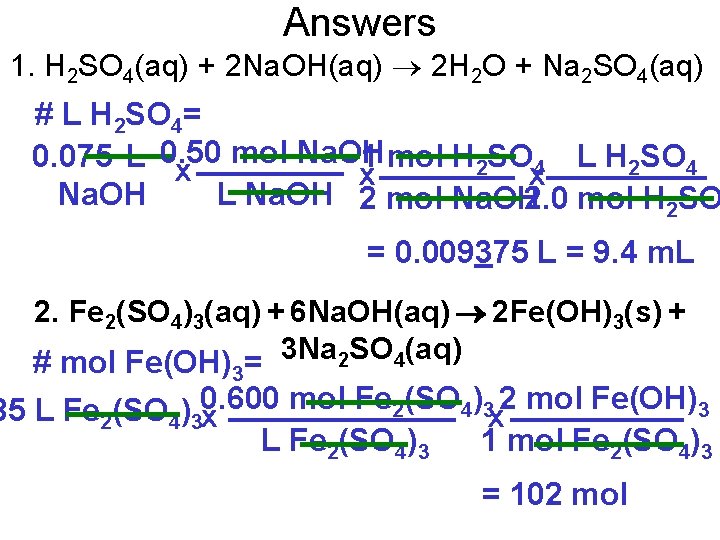
Answers 1. H 2 SO 4(aq) + 2 Na. OH(aq) 2 H 2 O + Na 2 SO 4(aq) # L H 2 SO 4= mol Na. OH 0. 075 L 0. 50 1 mol H SO L H SO 2 4 x x x L Na. OH 2 mol Na. OH 2. 0 mol H 2 SO = 0. 009375 L = 9. 4 m. L 2. Fe 2(SO 4)3(aq) + 6 Na. OH(aq) 2 Fe(OH)3(s) + 3 Na SO (aq) 2 4 # mol Fe(OH) = 3 mol Fe 2(SO 4)3 2 mol Fe(OH)3 85 L Fe 2(SO 4)30. 600 x x L Fe 2(SO 4)3 1 mol Fe 2(SO 4)3 = 102 mol

3. 2 Na. OH(aq) + Zn. Cl 2(aq) Zn(OH)2(s) + # g Zn(OH)2= 2 Na. Cl(aq) = 6. 21 g mol Na. OH 1 mol Zn(OH) 99. 40 g 0. 05002. 50 2 x x x L Na. OH 2 mol Na. OH 1 mol Zn(OH) L Na. OH Zn(OH) 2 2 4 a. 3 Ag. NO 3(aq) + K 3 PO 4(aq) Ag 3 PO 4(s) + 3 KNO 3(aq) # L Ag. NO 3 = = 0. 1875 L = 0. 19 L 0. 025 0. 50 mol K 3 PO 34 mol Ag. NO 3 L Ag. NO 3 x x x L K 3 PO 4 1 mol K 3 PO 40. 20 mol Ag. NO 4 b. 3 Ag. NO 3(aq) + K 3 PO 4(aq) Ag 3 PO 4(s) + 3 KNO 3(aq) # g Ag 3 PO 4= = 5. 2 g mol K 3 PO 14 mol Ag 3 PO 4 418. 58 g 0. 025 0. 50 x x x L K 3 PO 4 1 mol Ag. Ag 3 PO 4 4 3 PO

5. Al(NO 3)3(aq) + 3 Na. OH(aq) Al(OH)3(s) + 3 Na. NO 3(aq) #g Al(OH)3=0. 500 mol 0. 550 x L L Al(NO 3)3 1 mol Al(OH)3 77. 98 g x x 1 mol 1 Al(OH) mol 3 Al(NO Al(OH) = 3)321. 4 g Al(OH) 3 3 #g Al(OH)1. 50 0. 240 mol Na. OH 1 mol Al(OH) 77. 98 g Al(OH) 3= 3 x x x L Na. OH 3 mol L Na. OH 1 mol Na. OH = 9. 36 Al(OH) g Al(OH) 3 3 For more lessons, visit www. chalkbored. com
 Gram to gram conversion
Gram to gram conversion Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Phosphorus + oxygen equation
Phosphorus + oxygen equation Stoichiometry mole-mole
Stoichiometry mole-mole Mole mole factor
Mole mole factor Mol si
Mol si Mole mass and mole volume relationships
Mole mass and mole volume relationships Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Lesson 92 mole tunnel stoichiometry answers
Lesson 92 mole tunnel stoichiometry answers Vole vs mole
Vole vs mole Stoichiometry island diagram
Stoichiometry island diagram Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật