STATES OF MATTER EXPANSION and CONTRACTION of LIQUIDS













- Slides: 23

STATES OF MATTER

EXPANSION and CONTRACTION of LIQUIDS and GASES

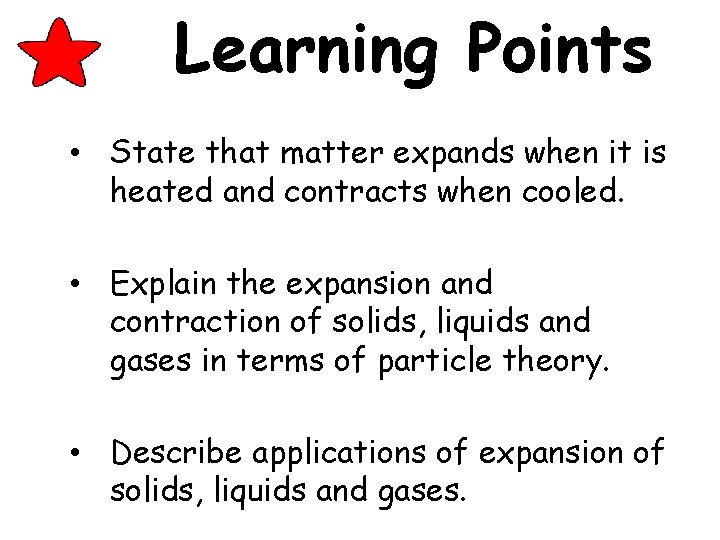
Learning Points • State that matter expands when it is heated and contracts when cooled. • Explain the expansion and contraction of solids, liquids and gases in terms of particle theory. • Describe applications of expansion of solids, liquids and gases.

Conduction of heat: If you ever go camping and hold a frying pan with a metal handle, you will soon feel the handle becoming hot, this is because metal is a good conductor of heat, i. e. it allows heat to flow through it. (The vibration of particles allows energy to be transferred) To prevent your hand from burning, fry-pan manufacturers put a plastic coating over the metal handle and the handle won’t feel hot because plastic and wood are poor conductors of heat, which we call insulators. conductor of heat: is something that can transfer heat easily Insulator of heat: is something that don’t transfer heat easily.

Expansion & contraction of liquids Mercury and alcohol are liquids that expand contract in thermometers.

Expansion and contraction: Solids, liquids and gases all expand (get larger) when they are heated, and therefore occupy more space. When they are cooled they contract (get smaller) and occupy less space. In solids particles vibrated in fixed positions, as the solid is heated up the particles vibrate more violently and they move further apart (as the particles need room to move), as a result the solid as a whole expand. The opposite happens when a solid is cooled down. Liquids and gases are exactly the same. Water is an exception, it expands when it freezes.

EXPERIMENT AIM: To find out what happens to liquids when they are heated and cooled.

INSTRUCTIONS 1. Fill two test-tubes to near the top with the red coloured water provided. 2. Place the tops on the test-tubes and secure them using a clamp-stand.

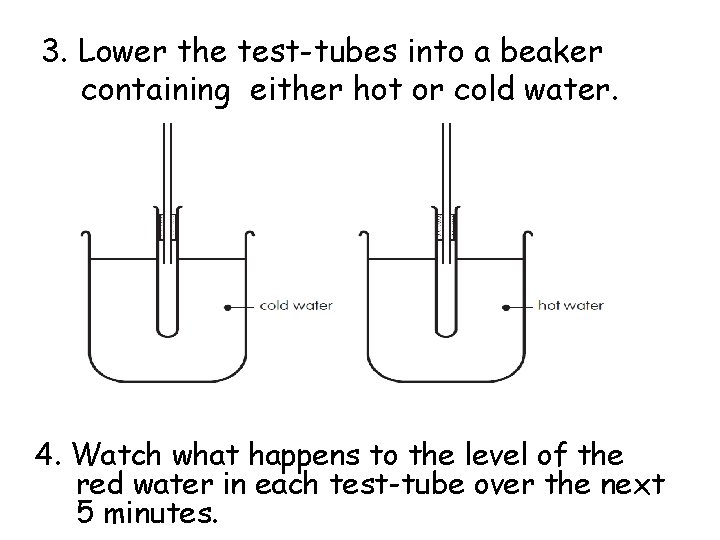
3. Lower the test-tubes into a beaker containing either hot or cold water. 4. Watch what happens to the level of the red water in each test-tube over the next 5 minutes.

WHAT DID YOU FIND OUT?

SOME QUESTIONS? Q. Can you explain why liquids expand when they are heated and contract when they are cooled? Q. What happens to how the particles in the red liquid are arranged when they are heated and then cooled?

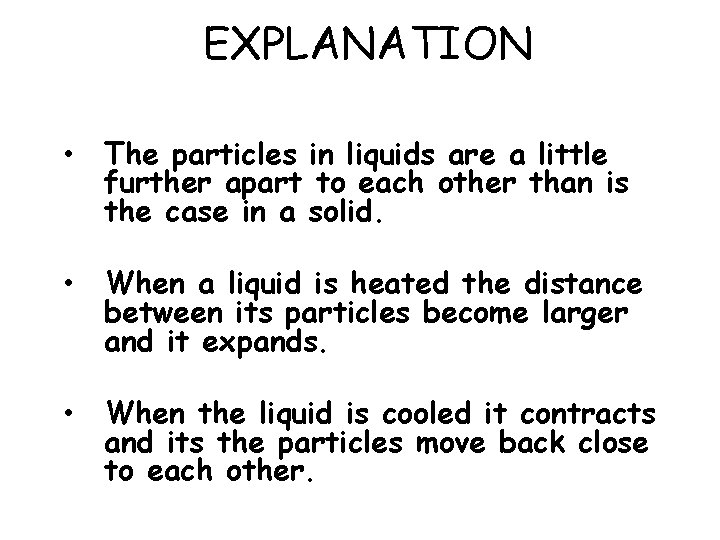
EXPLANATION • The particles in liquids are a little further apart to each other than is the case in a solid. • When a liquid is heated the distance between its particles become larger and it expands. • When the liquid is cooled it contracts and its the particles move back close to each other.

Expansion & contraction of gases Air expands when heated (becoming less dense) so it floats This is how hot air balloons work.

Questions What is a conductor of heat? What is an insulator of heat? Explain how expansion happens when something is heated. Explain how contraction happens when something is cooled.

EXPERIMENT AIM: To find out what happens to gases when they are heated and cooled.

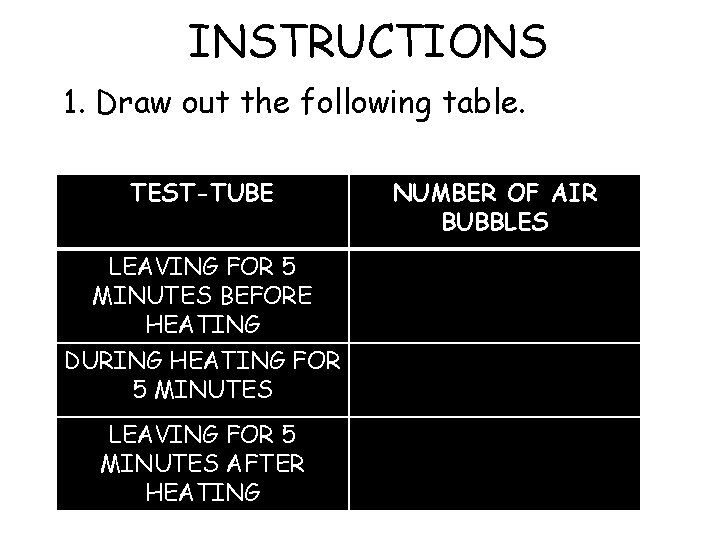
INSTRUCTIONS 1. Draw out the following table. TEST-TUBE LEAVING FOR 5 MINUTES BEFORE HEATING DURING HEATING FOR 5 MINUTES LEAVING FOR 5 MINUTES AFTER HEATING NUMBER OF AIR BUBBLES

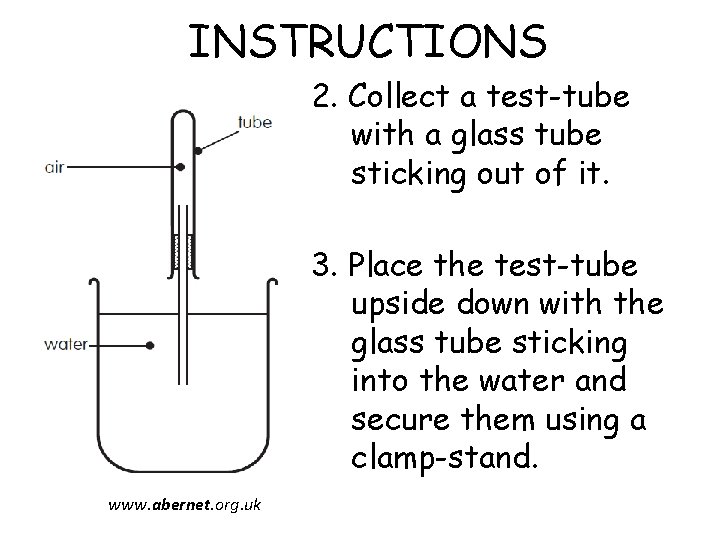
INSTRUCTIONS 2. Collect a test-tube with a glass tube sticking out of it. 3. Place the test-tube upside down with the glass tube sticking into the water and secure them using a clamp-stand. www. abernet. org. uk

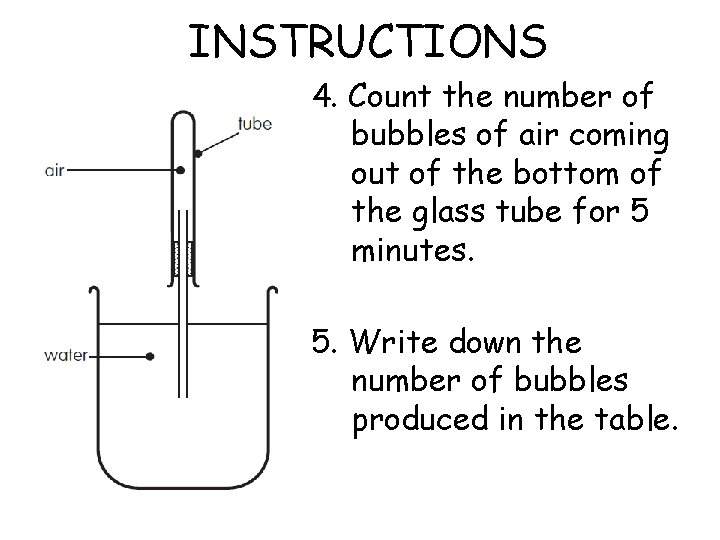
INSTRUCTIONS 4. Count the number of bubbles of air coming out of the bottom of the glass tube for 5 minutes. 5. Write down the number of bubbles produced in the table.

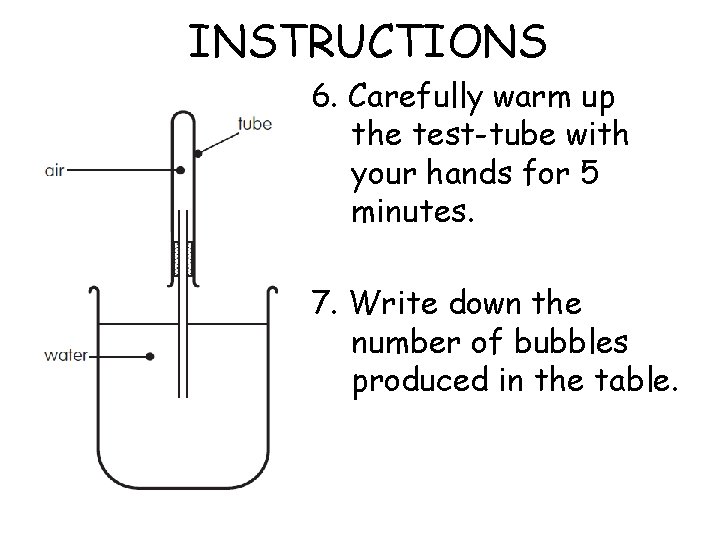
INSTRUCTIONS 6. Carefully warm up the test-tube with your hands for 5 minutes. 7. Write down the number of bubbles produced in the table.

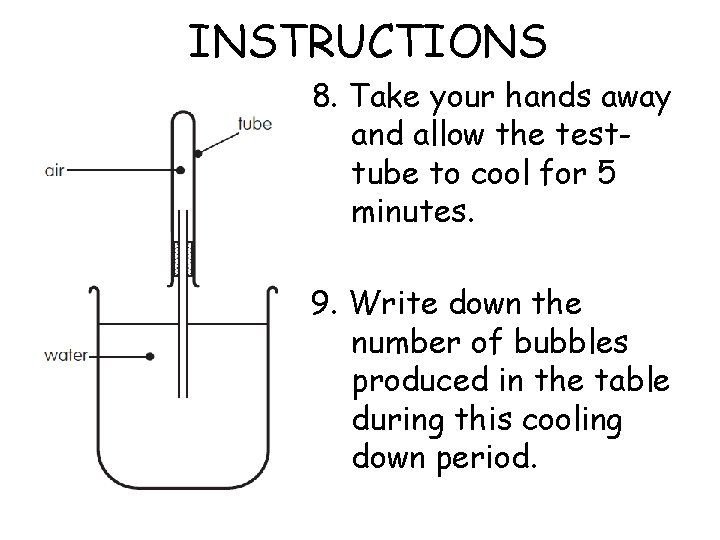
INSTRUCTIONS 8. Take your hands away and allow the testtube to cool for 5 minutes. 9. Write down the number of bubbles produced in the table during this cooling down period.

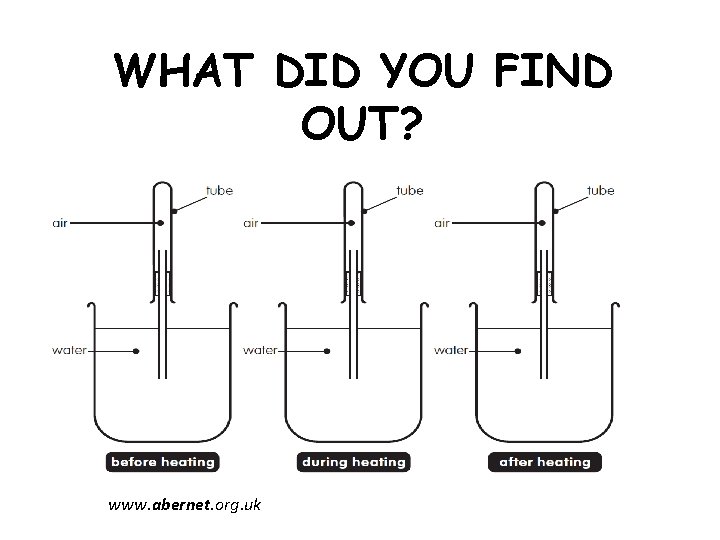
WHAT DID YOU FIND OUT? www. abernet. org. uk

WHAT DID YOU FIND OUT? • The heat from the hands made the air in the test tube heat up and expand. The expanded air pushed out of the test-tube and into the water as bubbles. • When the hot hands were taken away, the air in the test tube became cooler and the air began to cool. • The space in the test-tube taken up by the air that escaped during heating was replaced by water moving into the test-tube.

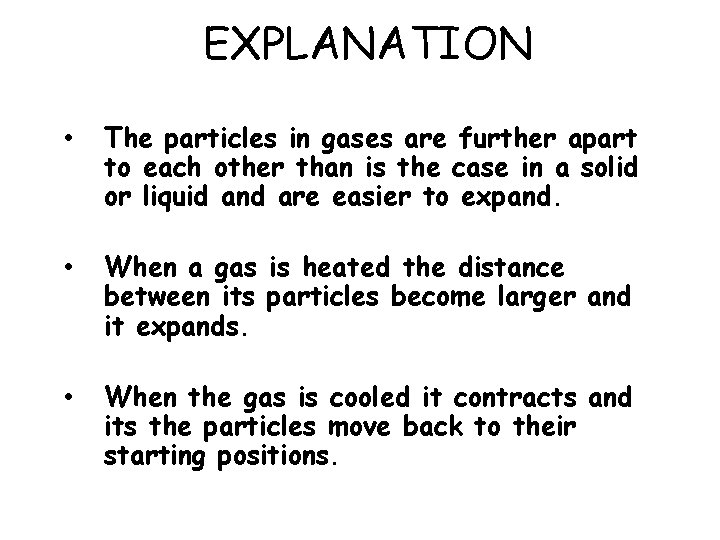
EXPLANATION • The particles in gases are further apart to each other than is the case in a solid or liquid and are easier to expand. • When a gas is heated the distance between its particles become larger and it expands. • When the gas is cooled it contracts and its the particles move back to their starting positions.
 Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Smooth muscle cell
Smooth muscle cell Expansion and contraction weathering
Expansion and contraction weathering Expansion and contraction
Expansion and contraction Thermal expansion and contraction examples
Thermal expansion and contraction examples Horizontal contraction/expansion matrix
Horizontal contraction/expansion matrix Thermal expansion examples
Thermal expansion examples Thermometer thermal expansion
Thermometer thermal expansion Water expands when heated
Water expands when heated Expansion of the united states of america 1607 to 1853 map
Expansion of the united states of america 1607 to 1853 map Particle arrangement of plasma
Particle arrangement of plasma Gray matter vs white matter
Gray matter vs white matter Gyrus and sulcus function
Gyrus and sulcus function Gray matter and white matter
Gray matter and white matter Ncl caudatus
Ncl caudatus Phases of matter foldable
Phases of matter foldable Four states of matter
Four states of matter Four states of matter
Four states of matter States of matter
States of matter Which state of matter has the most thermal energy
Which state of matter has the most thermal energy Changing state
Changing state Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter Solids liquids and gases venn diagram
Solids liquids and gases venn diagram