Solutions solutions are homogeneous mixtures made up of



- Slides: 18

Solutions -_____ solutions are _______ homogeneous mixtures made up of two or more ______; substances the ____, solute which _____, dissolves and the ____, solvent which does the ______ dissolving -generally, the ____ solvent is the substance with _____ smaller and numerous particles in the _____, solution while the more _____ solute usually has the _______, larger less _____ numerous particles ____ of the two substances in the _____ solution -_____ solutions can be ______, solids _______, liquids or ______, gases but most often are ____, liquids and ____ liquid _____ solutions are most aqueous since the most common ____ solvent is ______ water often ____, I. Solvation in Aqueous Solutions -_____ solvation is the process of surrounding _______ solute particles with ____ solvent particles to form solution a _____ -solvation done by ______ water is called _____ hydration

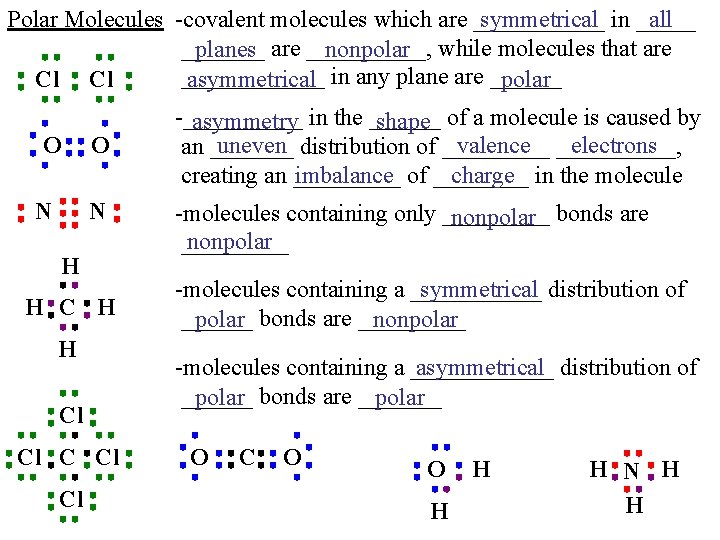
Polar Molecules -covalent molecules which are ______ symmetrical in _____ all _______ planes are _____, nonpolar while molecules that are ______ Cl Cl asymmetrical in any plane are ______ polar O N H H Cl Cl Cl -_____ asymmetry in the ______ shape of a molecule is caused by uneven distribution of _____ valence _____, electrons an _______ imbalance of ____ charge in the molecule creating an _____ -molecules containing only _____ nonpolar bonds are nonpolar _____ -molecules containing a ______ symmetrical distribution of ______ polar bonds are _____ nonpolar -molecules containing a ______ asymmetrical distribution of ______ polar bonds are _______ polar O C O O H H H N H H

Polar Molecules -_____ polarity is often created by _____ unshared _______ pairs of ____ valence _____ electrons weak attractions between the molecules -the relatively ________ intermolecular of covalent compounds are called ________, forces the ____ weakest of which are called _____ dispersion or _______ London ____ forces partial ____ poles of ______ polar molecules create -the ____ intermolecular forces called _______dipole stronger __________ dipole ____, forces the most _____ powerful of which are called _____ Hydrogen ______ bonds -_______ intermolecular _______ forces affect the _____ physical ______ properties of _____ covalent _____ compounds like ____ melting ______, point _______ boiling ______, point and _____ solubility

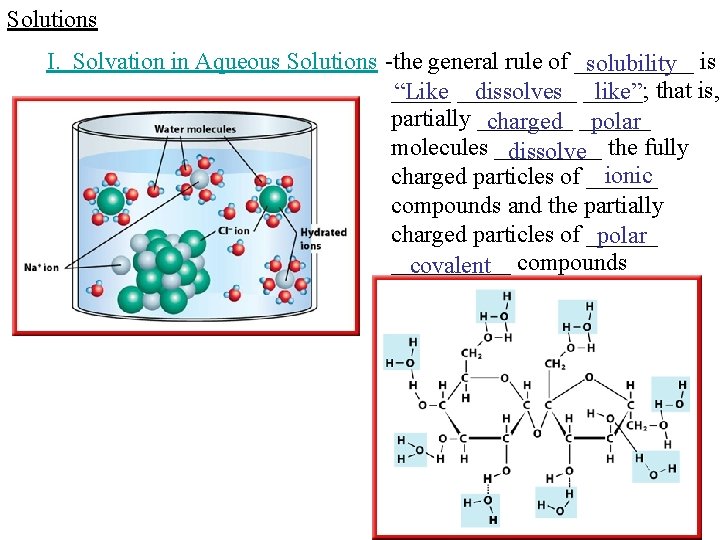
Solutions I. Solvation in Aqueous Solutions -the general rule of _____ solubility is _____ “Like _____ dissolves _____; like” that is, partially ____ charged ______ polar molecules _____ dissolve the fully ionic charged particles of ______ compounds and the partially charged particles of ______ polar _____ covalent compounds

Solutions I. Solvation in Aqueous Solutions A. Factors Affecting -the _____ solvation _____ rate is affected by Solvation Rate three factors which _____ increase the rate of ______ of ____ collision solute and _______ solvent particles -_____, agitation ______ increasing the ____ surface _____ area of the ____, solute and _____ increasing temperature of the ____ solvent all the ______ increase the rate of solvation ____ B. Heat of Solution -if the energy required to overcome the _____ attractive forces between _______ solute and ____ solvent particles is _______ greater than the released during the mixing of energy _____ the particles, _____ solvation is _____, endothermic but _____ solvation is _____ exothermic when more released than _____ required energy is _____

Solutions II. Solubility -_____ solubility is the ____ maximum amount of ______ solute that will ____ dissolve in a given amount of _______ solvent at a specific ______ temperature and _____ pressure -_____ saturated solutions contain the _____ maximum amount of dissolved _______ solute for a given amount of ____ solvent at a given ______ temperature and ____; pressure ______ unsaturated supersaturated solutions contain less than that, and ______ solutions contain more than that

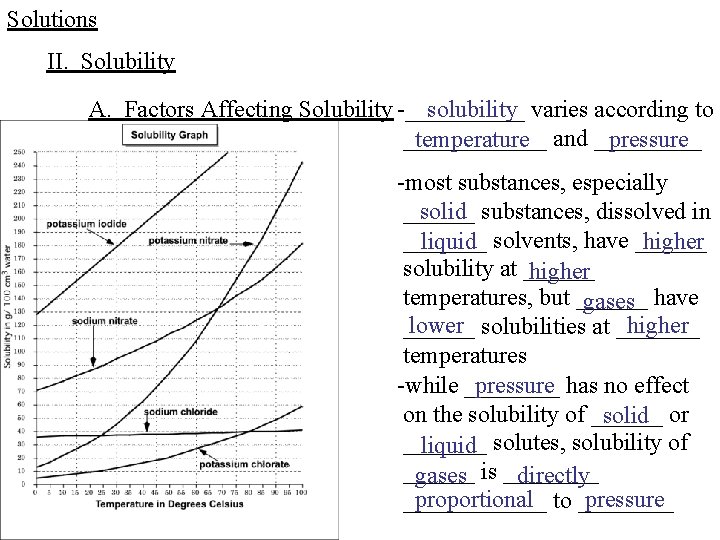
Solutions II. Solubility A. Factors Affecting Solubility -_____ solubility varies according to ______ temperature and _____ pressure -most substances, especially solid substances, dissolved in _______ liquid solvents, have ______ higher solubility at ______ higher temperatures, but ______ gases have lower solubilities at _______ higher ______ temperatures -while ____ pressure has no effect on the solubility of ______ solid or _______ liquid solutes, solubility of ______ gases is ____ directly proportional to ____ pressure ______

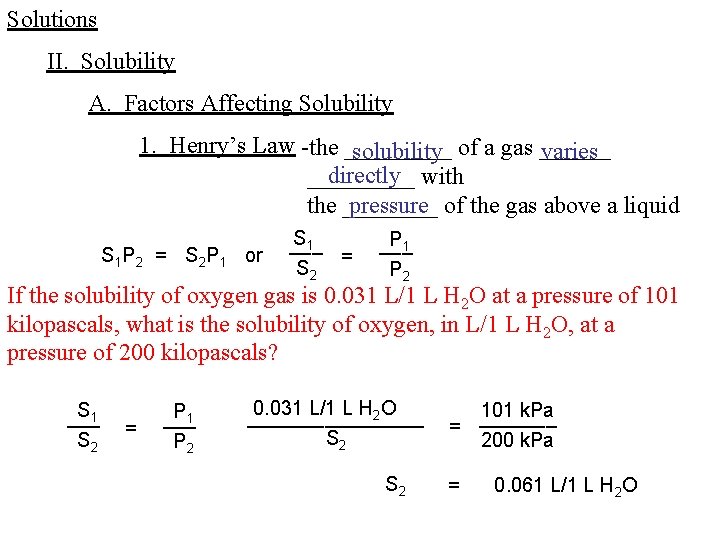
Solutions II. Solubility A. Factors Affecting Solubility 1. Henry’s Law -the _____ solubility of a gas ______ varies directly with _____ pressure of the gas above a liquid the ____ S 1 P 2 = S 2 P 1 or S 1 ___ = S 2 P 1 ___ P 2 If the solubility of oxygen gas is 0. 031 L/1 L H 2 O at a pressure of 101 kilopascals, what is the solubility of oxygen, in L/1 L H 2 O, at a pressure of 200 kilopascals? S 1 ___ S 2 = P 1 ___ P 2 0. 031 L/1 L H 2 O ________ S 2 101 k. Pa _______ = 200 k. Pa = 0. 061 L/1 L H 2 O

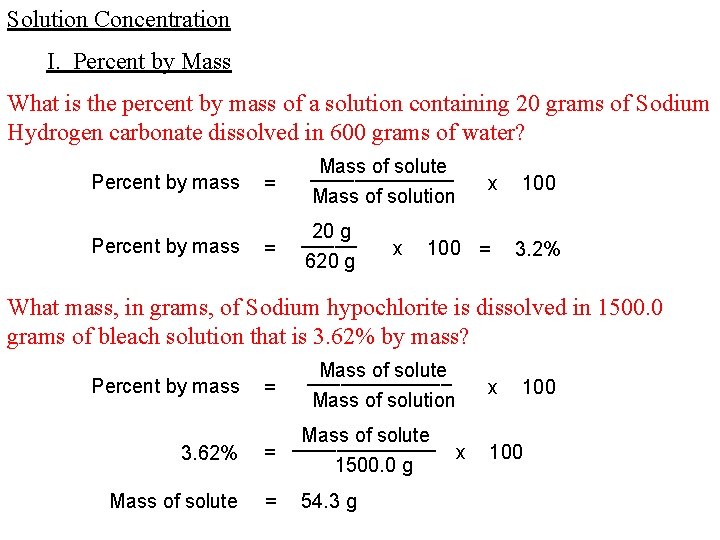
Solution Concentration I. Percent by Mass What is the percent by mass of a solution containing 20 grams of Sodium Hydrogen carbonate dissolved in 600 grams of water? Percent by mass = = Mass of solute _______ Mass of solution 20 g _____ 620 g x x 100 = 100 3. 2% What mass, in grams, of Sodium hypochlorite is dissolved in 1500. 0 grams of bleach solution that is 3. 62% by mass? Percent by mass 3. 62% Mass of solute = Mass of solute _______ Mass of solution Mass of solute _______ = x 1500. 0 g = 54. 3 g x 100

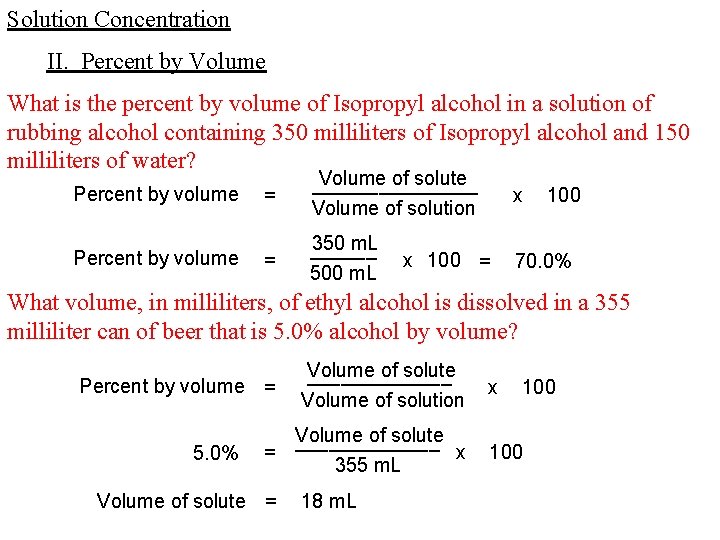
Solution Concentration II. Percent by Volume What is the percent by volume of Isopropyl alcohol in a solution of rubbing alcohol containing 350 milliliters of Isopropyl alcohol and 150 milliliters of water? Percent by volume = Volume of solute ________ Volume of solution x = 350 m. L ______ 500 m. L 70. 0% x 100 = 100 What volume, in milliliters, of ethyl alcohol is dissolved in a 355 milliliter can of beer that is 5. 0% alcohol by volume? Percent by volume = 5. 0% Volume of solute _______ Volume of solution Volume of solute _______ = x 355 m. L Volume of solute = 18 m. L x 100

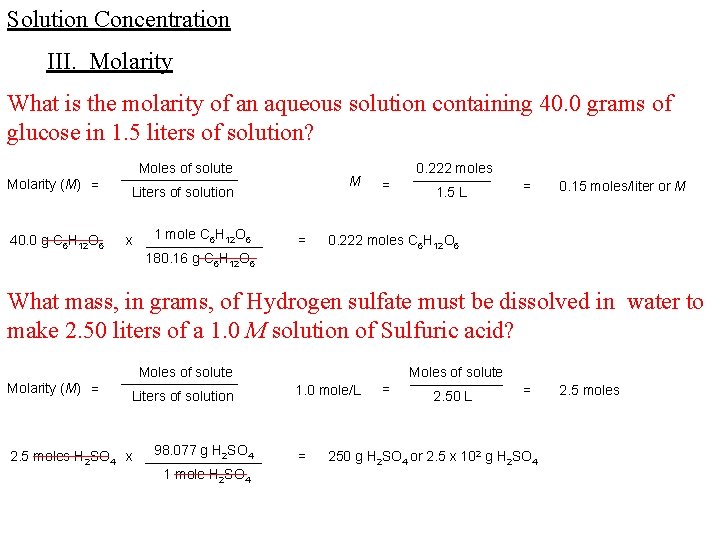
Solution Concentration III. Molarity What is the molarity of an aqueous solution containing 40. 0 grams of glucose in 1. 5 liters of solution? Molarity (M) = 40. 0 g C 6 H 12 O 6 Moles of solute ________ Liters of solution 1 mole C 6 H 12 O 6 x ________ 180. 16 g C 6 H 12 O 6 M = = 0. 222 moles _____ 1. 5 L = 0. 15 moles/liter or M 0. 222 moles C 6 H 12 O 6 What mass, in grams, of Hydrogen sulfate must be dissolved in water to make 2. 50 liters of a 1. 0 M solution of Sulfuric acid? Molarity (M) = Moles of solute ________ Liters of solution 98. 077 g H 2 SO 4 2. 5 moles H 2 SO 4 x ________ 1 mole H 2 SO 4 1. 0 mole/L = = Moles of solute ______ 2. 50 L = 250 g H 2 SO 4 or 2. 5 x 102 g H 2 SO 4 2. 5 moles

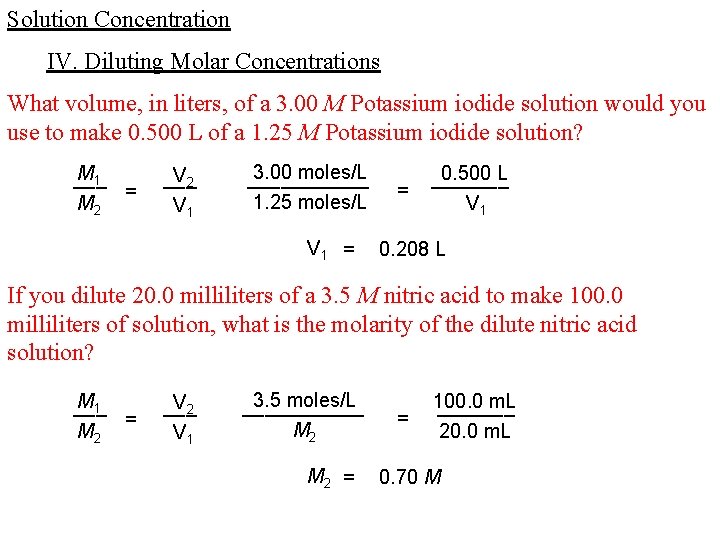
Solution Concentration IV. Diluting Molar Concentrations What volume, in liters, of a 3. 00 M Potassium iodide solution would you use to make 0. 500 L of a 1. 25 M Potassium iodide solution? M 1 ___ = M 2 V 2 ___ V 1 3. 00 moles/L ______ 1. 25 moles/L V 1 = = 0. 500 L _______ V 1 0. 208 L If you dilute 20. 0 milliliters of a 3. 5 M nitric acid to make 100. 0 milliliters of solution, what is the molarity of the dilute nitric acid solution? M 1 ___ = M 2 V 2 ___ V 1 3. 5 moles/L ______ M 2 = = 100. 0 m. L _______ 20. 0 m. L 0. 70 M

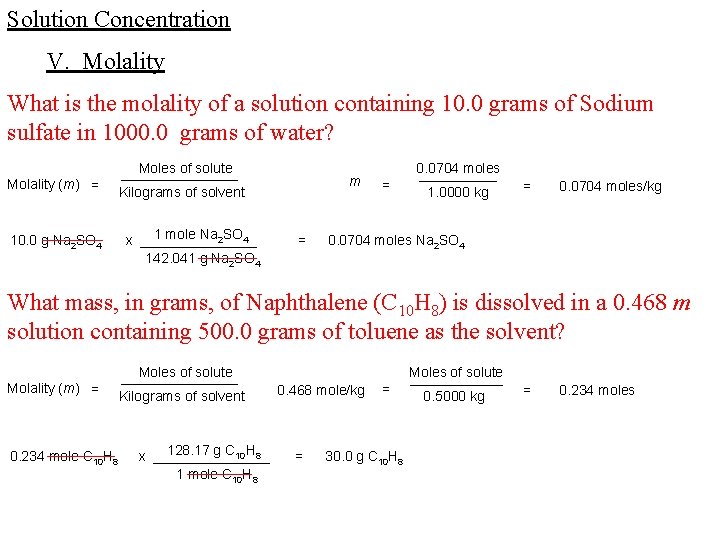
Solution Concentration V. Molality What is the molality of a solution containing 10. 0 grams of Sodium sulfate in 1000. 0 grams of water? Molality (m) = 10. 0 g Na 2 SO 4 Moles of solute ________ Kilograms of solvent 1 mole Na 2 SO 4 x ________ 142. 041 g Na 2 SO 4 m = = 0. 0704 moles _____ 1. 0000 kg = 0. 0704 moles/kg 0. 0704 moles Na 2 SO 4 What mass, in grams, of Naphthalene (C 10 H 8) is dissolved in a 0. 468 m solution containing 500. 0 grams of toluene as the solvent? Molality (m) = 0. 234 mole C 10 H 8 Moles of solute ________ Kilograms of solvent 128. 17 g C 10 H 8 x ________ 1 mole C 10 H 8 0. 468 mole/kg = = 30. 0 g C 10 H 8 Moles of solute ______ 0. 5000 kg = 0. 234 moles

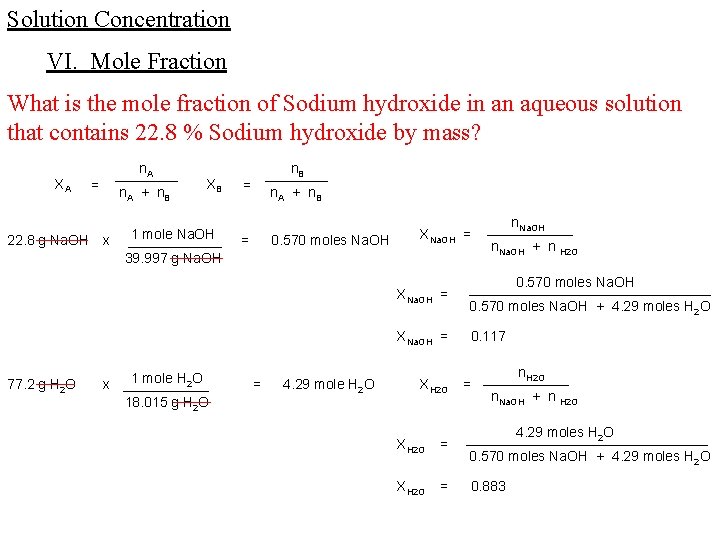
Solution Concentration VI. Mole Fraction What is the mole fraction of Sodium hydroxide in an aqueous solution that contains 22. 8 % Sodium hydroxide by mass? XA 22. 8 g Na. OH 77. 2 g H 2 O n. A ____ n. A + n B = x XB 1 mole Na. OH ______ 39. 997 g Na. OH 1 mole H 2 O x ______ 18. 015 g H 2 O n. B ____ n. A + n B = = 0. 570 moles Na. OH = 4. 29 mole H 2 O XNa. OH = n. Na. OH ______ n. Na. OH + n H 2 O XNa. OH = 0. 570 moles Na. OH ________________ 0. 570 moles Na. OH + 4. 29 moles H 2 O XNa. OH = 0. 117 XH 2 O n. H 2 O = ______ n. Na. OH + n H 2 O XH 2 O = 4. 29 moles H 2 O ________________ 0. 570 moles Na. OH + 4. 29 moles H 2 O XH 2 O = 0. 883

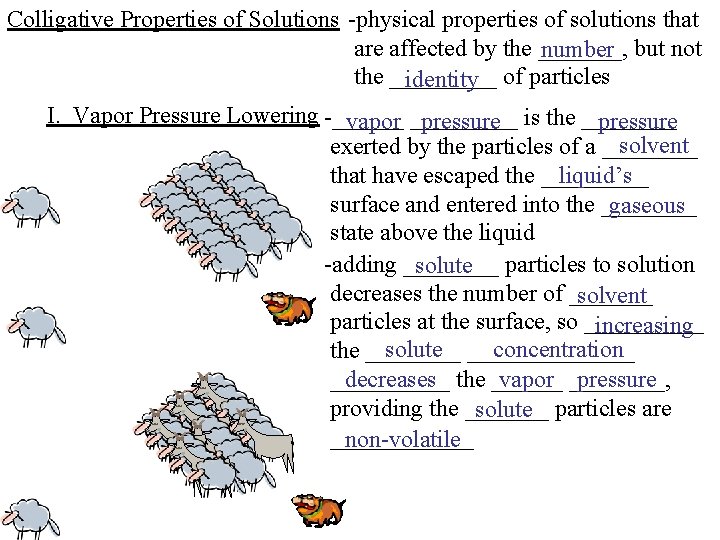
Colligative Properties of Solutions -physical properties of solutions that are affected by the _______, number but not the _____ identity of particles I. Vapor Pressure Lowering -______ vapor _____ pressure is the ____ pressure solvent exerted by the particles of a ____ liquid’s that have escaped the _____ surface and entered into the ____ gaseous state above the liquid -adding ____ solute particles to solution decreases the number of _______ solvent particles at the surface, so _____ increasing solute _______ concentration the ____ decreases the ______ vapor ____, pressure _____ providing the _______ solute particles are ______ non-volatile

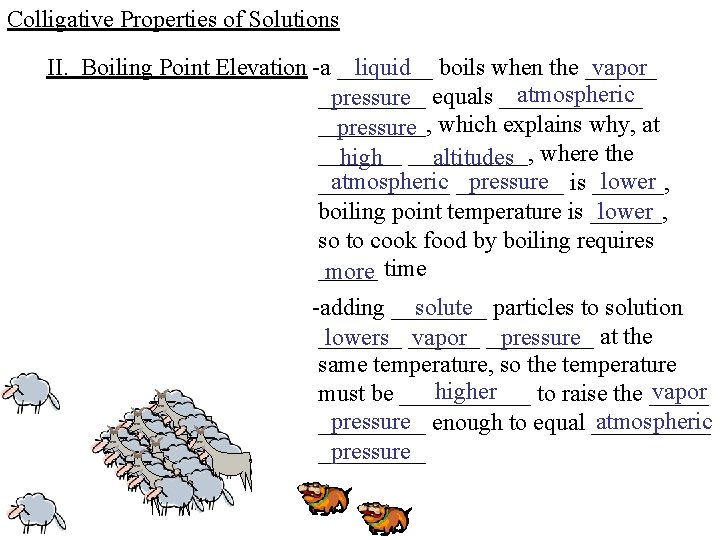
Colligative Properties of Solutions II. Boiling Point Elevation -a ____ liquid boils when the ______ vapor atmospheric _____ pressure equals ______, pressure which explains why, at _______ high _____, altitudes where the atmospheric _____ pressure is ______, lower ______ boiling point temperature is ______, lower so to cook food by boiling requires _____ more time -adding ____ solute particles to solution _______ lowers ______ vapor _____ pressure at the same temperature, so the temperature higher vapor must be ______ to raise the _____ pressure enough to equal _____ atmospheric _____ pressure _____

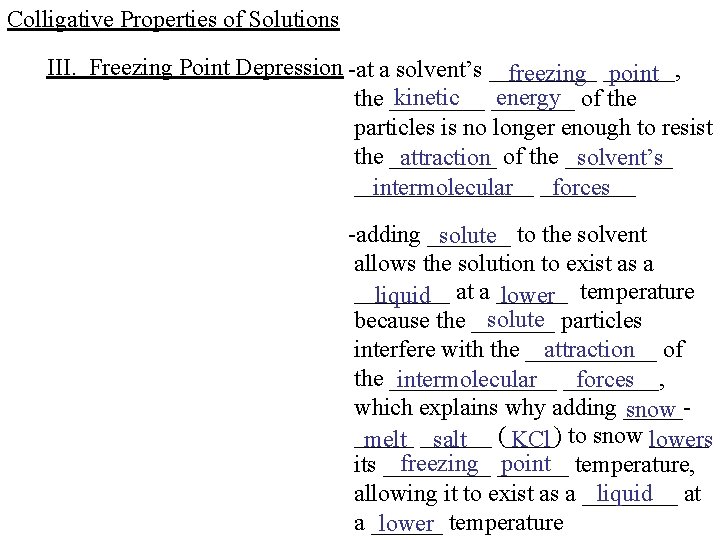
Colligative Properties of Solutions III. Freezing Point Depression -at a solvent’s _____ freezing ______, point kinetic _______ energy of the ____ particles is no longer enough to resist the _____ attraction of the _____ solvent’s ________ intermolecular ____ forces -adding _______ solute to the solvent allows the solution to exist as a ____ liquid at a ______ lower temperature solute particles because the _______ attraction of interfere with the ______________ intermolecular ____, forces which explains why adding _____snow _____ melt ______ salt (____) KCl to snow lowers freezing ______ point temperature, its _____ liquid at allowing it to exist as a ______ lower temperature

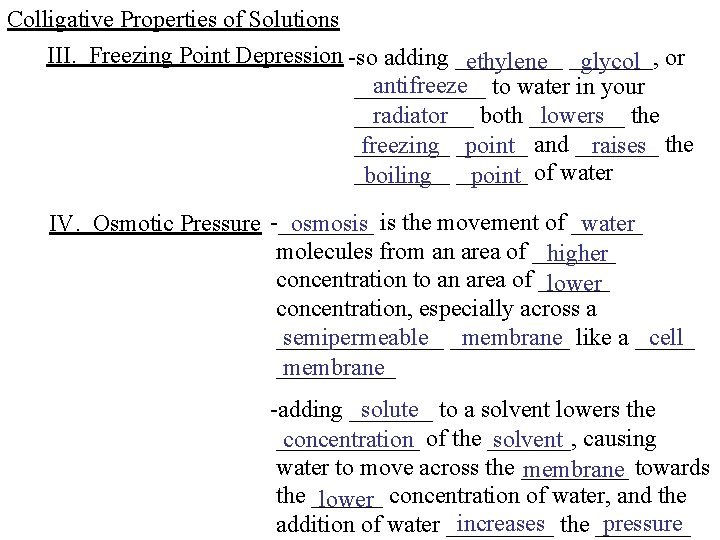
Colligative Properties of Solutions III. Freezing Point Depression -so adding _____ ethylene _______, glycol or antifreeze to water in your ______ radiator both ____ lowers the _____ freezing ______ point and _______ raises the ____ boiling ______ point of water IV. Osmotic Pressure -____ osmosis is the movement of ______ water molecules from an area of _______ higher concentration to an area of ______ lower concentration, especially across a semipermeable _____ membrane like a _____ cell _______ membrane solute to a solvent lowers the -adding ____________ concentration of the _______, solvent causing water to move across the _____ membrane towards the ______ lower concentration of water, and the increases the ____ pressure addition of water _____