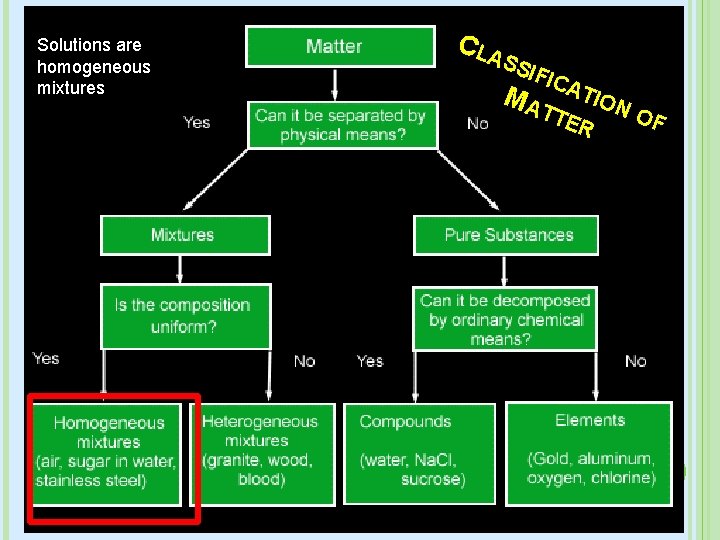
SOLUTIONS Solutions are homogeneous mixtures C LA SSI






















- Slides: 42

SOLUTIONS

Solutions are homogeneous mixtures C LA SSI FIC MA ATION TTE OF R

DEFINITIONS Solution - homogeneous mixture � (remember, the particles are so tiny they don’t settle out like in heterogeneous mixtures) Solute - substance being dissolved Solvent - present in greater amount

DEFINITIONS Solvent - H 2 O Solute - KMn. O 4

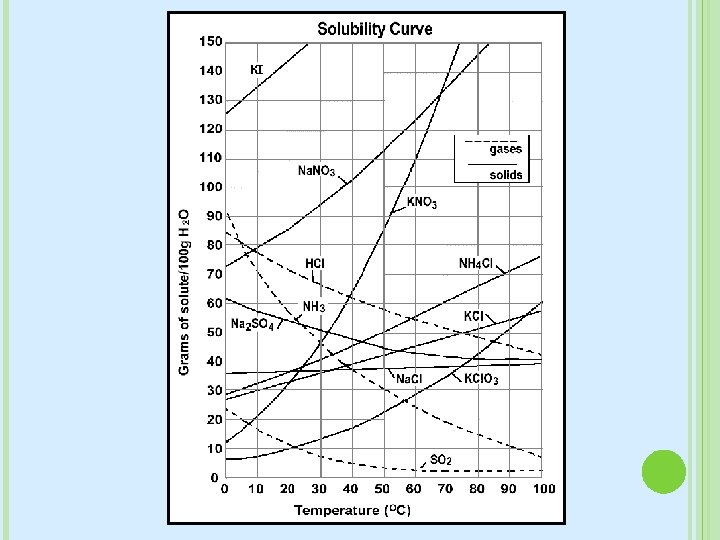
SOLUBILITY Solubility tells us how well something dissolves � Soluble: Dissolves � Insoluble: Doesn’t dissolve Solubility definition: � maximum grams of solute that will dissolve in 100 g of solvent at a given temperature � Related to the saturation of a solution

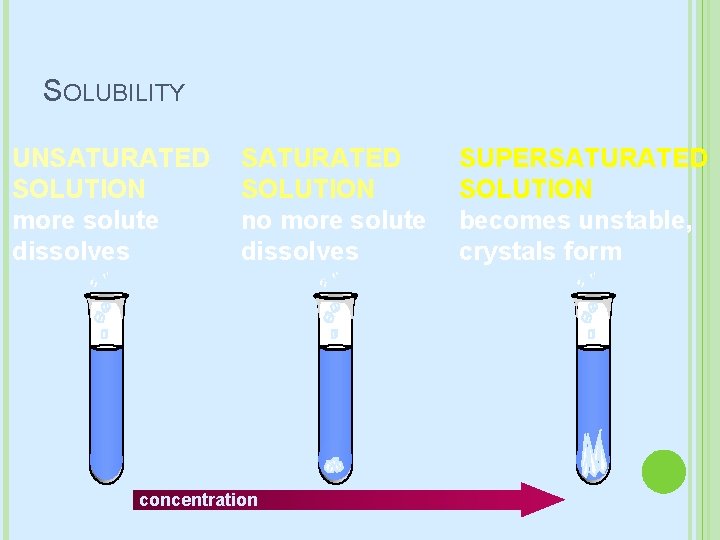
SATURATION Unsaturated: � Not full � More solute can be dissolved in the solvent Saturated � Full � More solute CANNOT be dissolved in the solvent Supersaturated � Over Full � More solute has been added than normal Usually because you’ve added heat to the solvent.

SOLUBILITY UNSATURATED SOLUTION more solute dissolves SATURATED SOLUTION no more solute dissolves concentration SUPERSATURATED SOLUTION becomes unstable, crystals form

FACTORS THAT AFFECT SOLUBILITY & DISSOLVING Surface Area � If the solute covers more surface area, it will dissolve faster Ex. Cube of sugar vs sugar particles Stirring � Stirring speeds up dissolving time Think of how long you would have to wait if you didn’t stir your chocolate syrup into your milk Temperature � Hotter temperatures= Faster � Hotter temperatures = More soluble � For gases, colder temperatures are more favorable


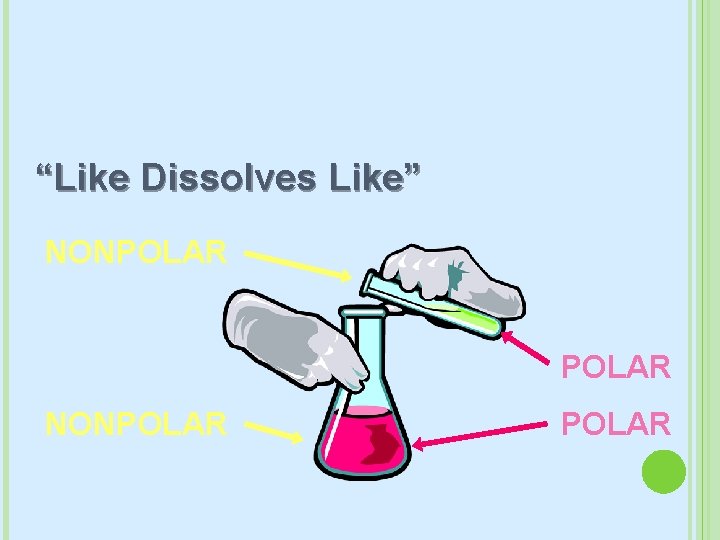
POLARITY (AGAIN) If a solute will dissolve in a solvent depends on the polarity Remember: Like Dissolves Like � Polar dissolves Polar � Non-Polar dissolves Non-Polar If things don’t mix then they have different polarities Some definitons � Miscible: Liquids that mix together � Immiscible: Liquids that don’t mix together

“Like Dissolves Like” NONPOLAR

COLLIGATIVE PROPERTIES properties that depend on the concentration of solute particles but not on their identity Boiling points and freezing points of solutions differ from those of pure solvent.

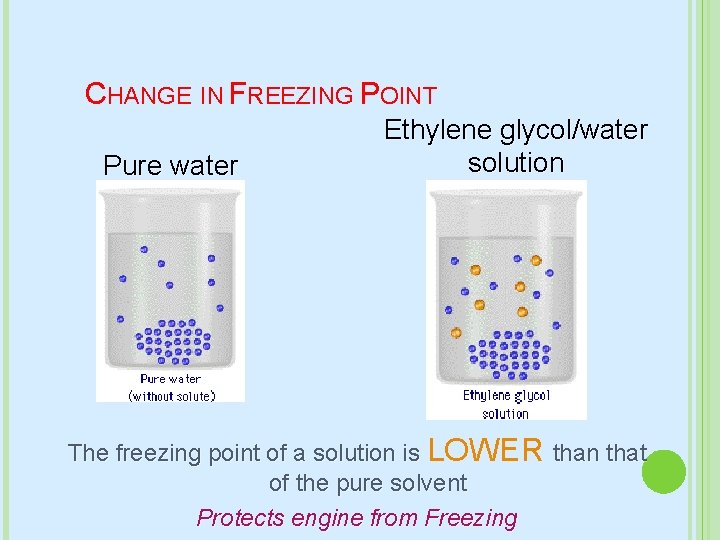
CHANGE IN FREEZING POINT Pure water Ethylene glycol/water solution The freezing point of a solution is LOWER than that of the pure solvent Protects engine from Freezing

CHANGE IN FREEZING POINT Common Applications of Freezing Point Depression Ethylene glycol – deadly to small animals Propylene glycol

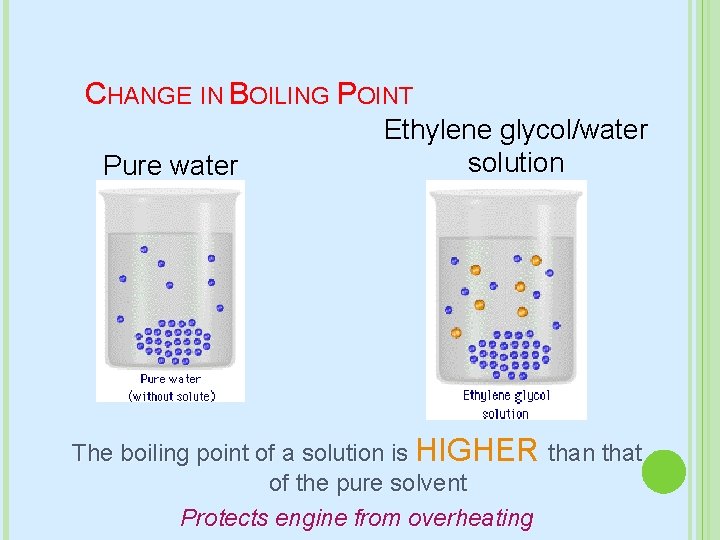
CHANGE IN BOILING POINT Pure water Ethylene glycol/water solution The boiling point of a solution is HIGHER than that of the pure solvent Protects engine from overheating

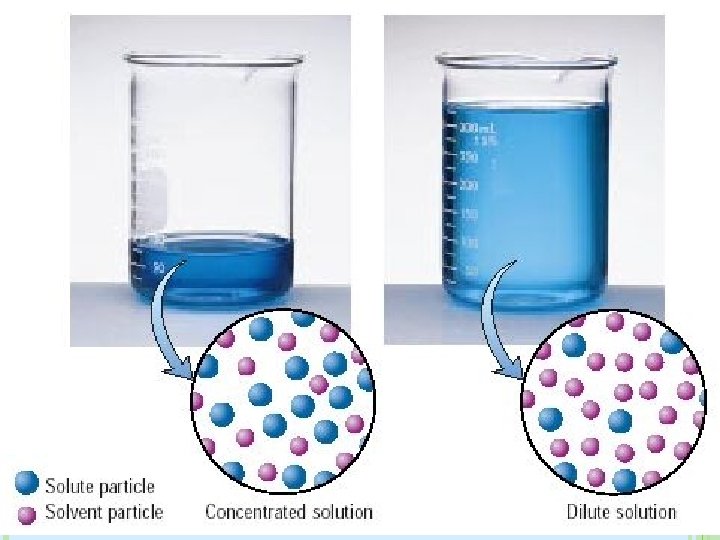
CONCENTRATION Concentration: The amount of solute dissolved in a given amount of solvent � Dilute= low concentration of solute � Concentrated= High concentration of solute Three ways to calculate concentration � Molarity (M) THE MOST COMMON!! � Percent Concenrtation (%m/v), (%m/m) , (%v/v) � Molality (m)

CONCENTRATED VS. DILUTE

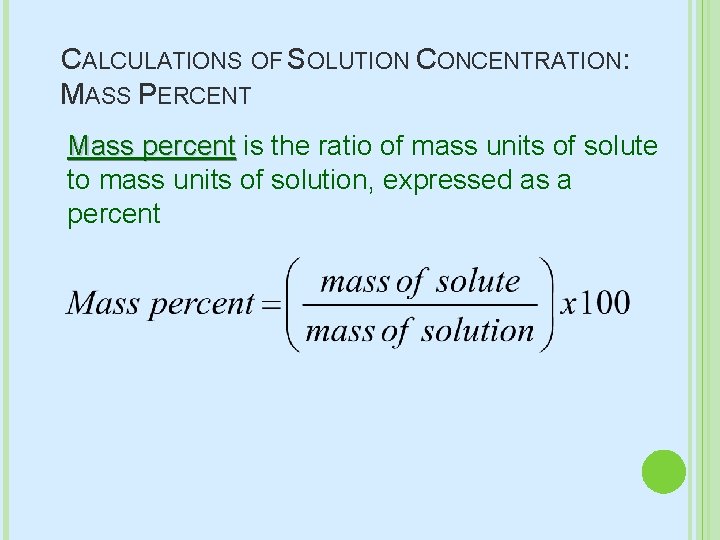
CALCULATIONS OF SOLUTION CONCENTRATION: MASS PERCENT Mass percent is the ratio of mass units of solute to mass units of solution, expressed as a percent

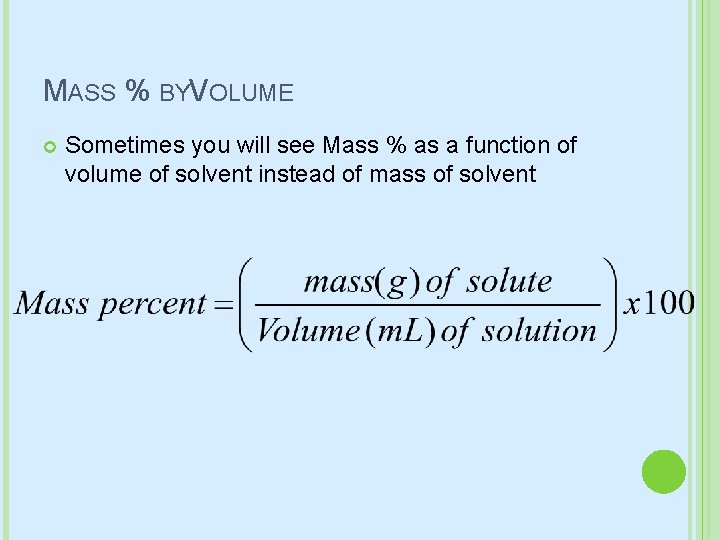
MASS % BYVOLUME Sometimes you will see Mass % as a function of volume of solvent instead of mass of solvent

A SIMPLIFYING ASSUMPTION 1 ml of water = 1 gram of water 1000 ml of water = 1 liter = 1000 grams Assume that solutions with water as the solvent have the density of pure water (1 m. L = 1 gram) �It’s not true, but it’s close enough

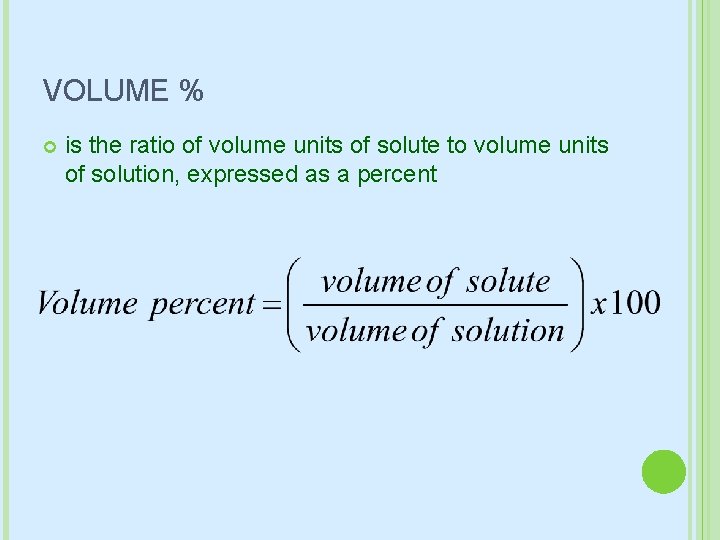
VOLUME % is the ratio of volume units of solute to volume units of solution, expressed as a percent

WHAT IS THE PERCENT CONCENTRATION OF 2. 7 G OF GLUCOSE IN 75 M L OF WATER?

85 M L OF ETHANOL IS DILUTED TO 250 L M OF SOLUTION. WHAT IS THE % CONCENTRATION OF ETHANOL?

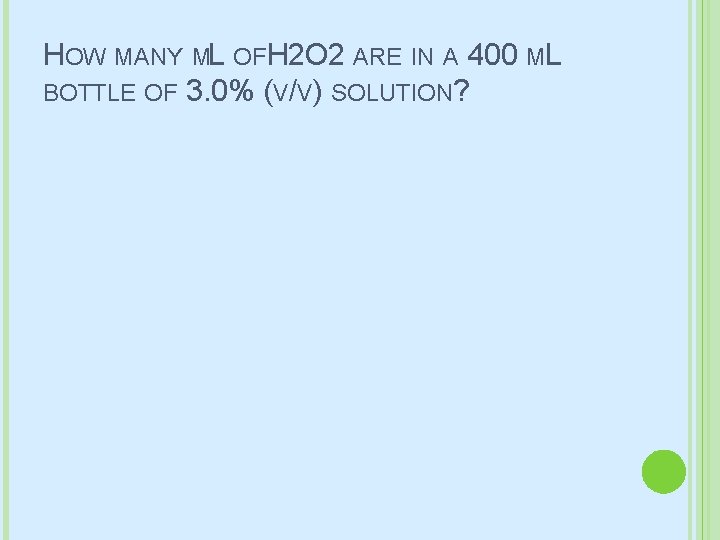
HOW MANY ML OFH 2 O 2 ARE IN A 400 ML BOTTLE OF 3. 0% (V/V) SOLUTION?

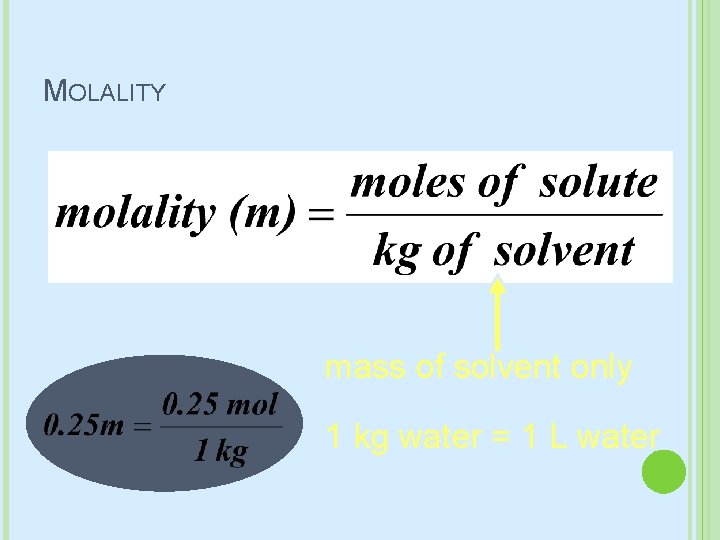
MOLALITY mass of solvent only 1 kg water = 1 L water

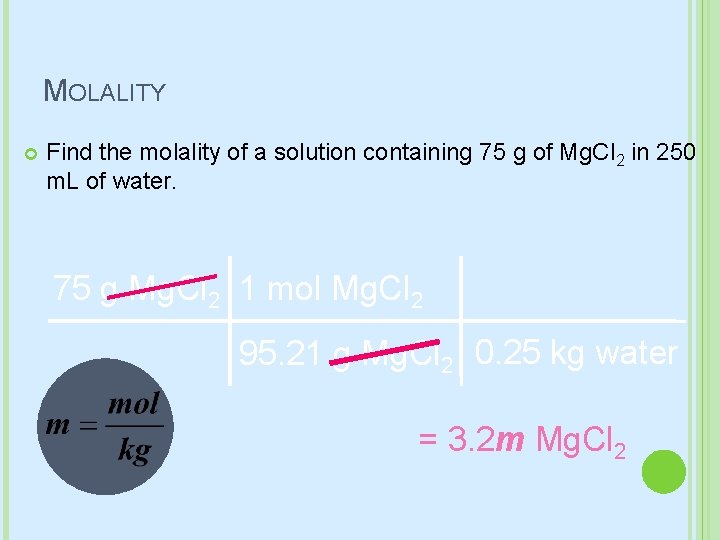
MOLALITY Find the molality of a solution containing 75 g of Mg. Cl 2 in 250 m. L of water. 75 g Mg. Cl 2 1 mol Mg. Cl 2 95. 21 g Mg. Cl 2 0. 25 kg water = 3. 2 m Mg. Cl 2

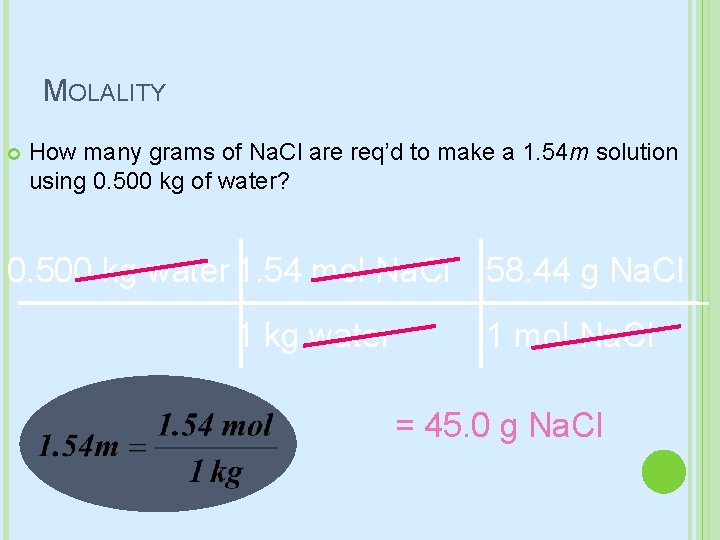
MOLALITY How many grams of Na. Cl are req’d to make a 1. 54 m solution using 0. 500 kg of water? 0. 500 kg water 1. 54 mol Na. Cl 1 kg water 58. 44 g Na. Cl 1 mol Na. Cl = 45. 0 g Na. Cl

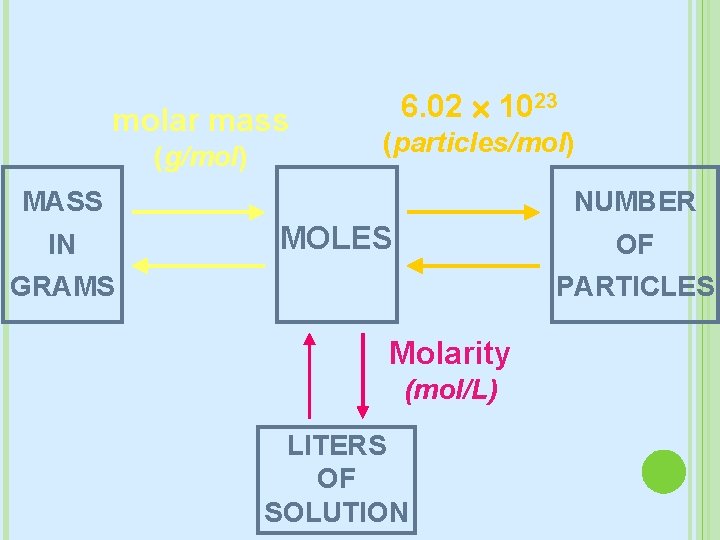
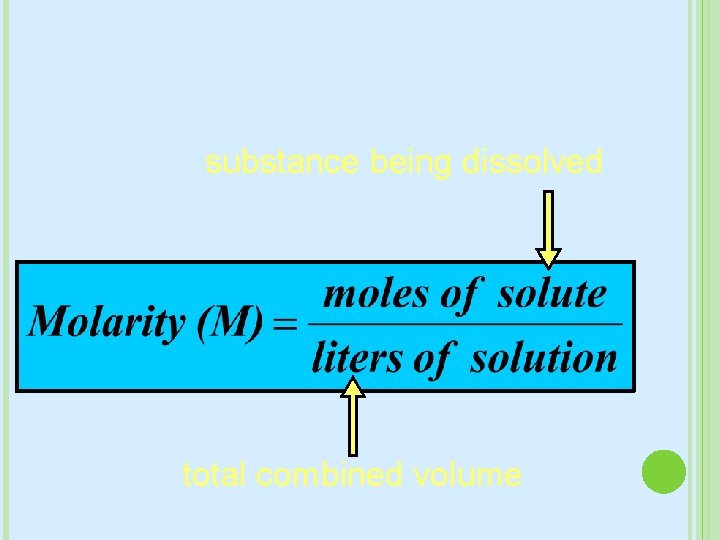
MOLARITY The most common way we measure concentration M = mol L Moles of solute per Liters of Solution (not just solvent, but amount solute+solvent) In some problems: � you may need to convert grams to moles first � You may need to convert m. L to L

molar mass (g/mol) 6. 02 1023 (particles/mol) NUMBER MASS IN MOLES OF PARTICLES GRAMS Molarity (mol/L) LITERS OF SOLUTION

substance being dissolved total combined volume

WHAT IS THE CONCENTRATION OF A SOLUTION MADE FROM 0. 2 MOLES OFKCL IN 0. 6 L OF WATER?

YOU HAVE 5. 50 L OF SOLUTION THAT CONTAINS 190 G OFNACL. WHAT IS THE MOLARITY OF THE SOLUTION?

HOW MUCH DISTILLED WATER (IN ML) WOULD BE REQUIRED TO MAKE A 0. 4 M SOLUTION USING 32 G OF NANO 3?

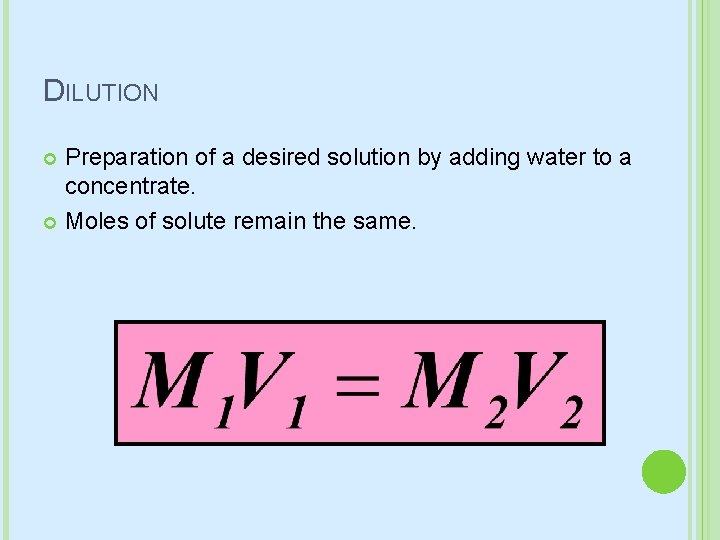
DILUTION Preparation of a desired solution by adding water to a concentrate. Moles of solute remain the same.


DILUTION What volume of 15. 8 M HNO 3 is required to make 250 m. L of a 6. 0 M solution? GIVEN: M 1 = 15. 8 M V 1 = ? M 2 = 6. 0 M V 2 = 250 m. L WORK: M 1 V 1 = M 2 V 2 (15. 8 M) V 1 = (6. 0 M)(250 m. L) V 1 = 95 m. L of 15. 8 M HNO 3

DILUTION How many milliliters of a stock (or standard) solution of 4. 00 M KI would you need to prepare 250 m. L of 0. 760 M KI?

DILUTION Suppose you need 250 m. L of a a 0. 20 M Na. Cl, but the only supply of sodium chloride you have is a solution of 1. 0 M Na. Cl. How do you prepare the required solution?

PREPARING SOLUTIONS • Weigh out a solid solute and dissolve in a given quantity of solvent. • Dilute a concentrated solution to give one that is less concentrated.

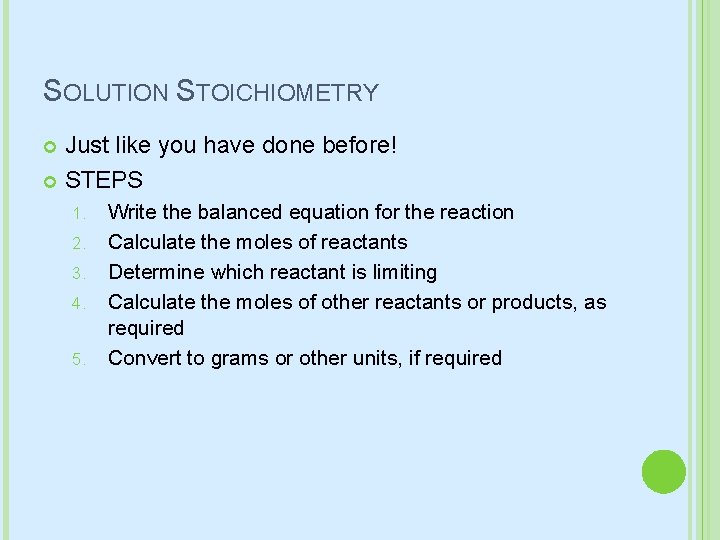
SOLUTION STOICHIOMETRY Just like you have done before! STEPS 1. 2. 3. 4. 5. Write the balanced equation for the reaction Calculate the moles of reactants Determine which reactant is limiting Calculate the moles of other reactants or products, as required Convert to grams or other units, if required

CALCULATE THE MASS OF SOLIDNACL THAT MUST BE ADDED TO A 1. 50 L OF A 0. 100 M AGNO 3 SOLUTION TO PRECIPITATE ALL OF THEAG+ IONS IN THE FORM OF AGCL. CALCULATE THE MASS OF AGCL FORMED. Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3

When Ba(NO 3)2 and K 2 Cr. O 4 react in aqueous solution, the yellow solid Ba. Cr. O 4 is formed. Calculate the mass of Ba. Cr. O 4 that forms when 3. 50 x 10 -3 mole of solid Ba(NO 3)2 is dissolved in 265 m. L of 0. 01000 M of K 2 Cr. O 4 solution